Introduction
Mycorrhizal arbuscular symbiosis is widespred in most economically important crops, and approximately 80 % of plant species establish this type of symbiosis (van-der-Heidjen et al., 2015). Within the ecosystems, this type of symbiosis is recognized as an adaptation mechanism; it increases the plant’s capacity to absorb nutrients and water, reduces pathogenic damages, and improving soil aggregates among other effects (Hamel and Strullu, 2006; Pozo and Azcón-Aguilar, 2007; Willis et al., 2013; Yang et al., 2014; Bagyara et al., 2015). However, it is a current challenge to achieve the proper management of this symbiosis through the inoculation of efficient strains in agricultural production.
In recent years, positive results have been obtained by the inoculation of efficient strains of arbuscular mycorrhizal fungi (AMF) in different crops and soil types in Cuba (Rivera et al., 2015). The integration of AMF in nutrient supply schemes, whether in mineral or organic-mineral fertilizers, has led to decrease in the quantities used, maintaining high yields and adequate nutritional status (Rivera et al., 2007; González et al., 2015; Ruiz-Sánchez et al., 2016).
The use of inoculated green manures, specifically Canavalia ensiformis L. (jackbean), in nutrient supply schemes has been successful in recent studies (Martín et al., 2013, Simó et al., 2016), its benefits have been associated not only with the incorporation and recycling of nutrients present in the biomass, but also with the effective mycorrhizal symbiosis present in inoculated Jackbean, which significantly increases mycorrhizal propagules in the soil, and behaves as an effective and economic way to mycorrhizal economic crops in succession.
The basis of the positive effect of permanence of the inoculant applied to the green manure is the generalist character of the strains of AMF used with the plant species, which means that an efficient strain for a given edaphic condition establishes an effective symbiosis with any crop dependent on arbuscular mycorrhization (Rivera et al., 2007). This effect also adds value to the use of green manures in agricultural production.
Although the importance of the soil environment and its pH have been established in the effectiveness of inoculation of AMF strains in different types of soils (Rivera et al., 2015), and several works have been developed in Jackbean as host (Martín et al., 2015; García et al., 2017), there are no previous reports that compare the efficiency of these strains when inoculated with crops in Calcaric Histosol soils of agricultural importance, with pH>7,5 that abound in the central and eastern regions of Cuba. Therefore, as part of the promotion of the joint use of mycorrhizal inoculants and Jackbean in Cuban agriculture, this work was developed with the objective of comparing the effectiveness of four strains of AMF inoculated in C. ensiformis seeds in Calcaric Histosol soils.
Materials and methods
The experiment was carried out between May and June of 2012 and 2013 with C. ensiformis L. and AMF in microplots located in the Department of Plant Science of the Instituto de Investigación de Cultivos de Raíces y Tubérculos Tropicales (INIVIT), located at 22º 35’ N, 80º 18’ W and 40 m.a.s.l., in the municipality of Santo Domingo, province of Villa Clara province, Cuba.
The dimensions of each microplots was 1 m2 by 0.60 m deep and were filled with Calcaric Histosol soil (WRB, 2014) from the INIVIT field # 20 and the 0-20 cm deep arable layer. The methodologies described by Paneque et al. (2010) were used for the characterization of agrochemicals (Table 1), the total content of mycorrhizal spore was measured by the wet sieving and decanting method according to Herrera et al. (1995).
Table 1 Agrochemical characteristics and initial spore content in the Calcaric Histosol soil (0-20 cm deep). Santo Domingo, province of Villa Clara, Cuba. 2012. Cuadro 1. Características agroquímicas y contenido inicial de esporas en el suelo Calcaric Histosol (0-20 cm de profundidad). Santo Domingo, provincia de Villa Clara, Cuba. 2012.
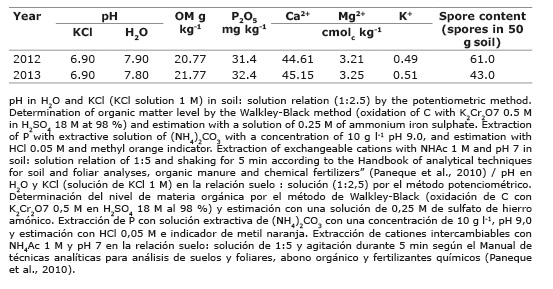
According to soil analysis, it showed a slightly alkaline pH, with high concentrations of exchangeable Ca2+, available phosphorus, as well as medium contents of interchangeable K+ and Mg2+, and low organic matter, the latter possibly related to degradation processes by the continuous cultivation. The contents of mycorrhizal spores were low and possibly due continuous systematic cultivation in the presence of high doses of mineral fertilizers.
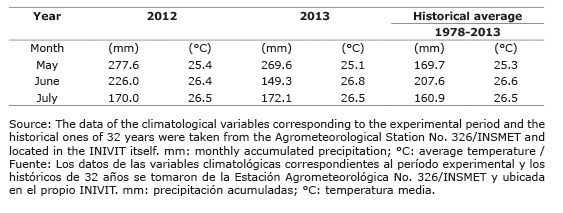
Monthly precipitation and average temperatures in the experimental area (Table 2) were adequate for the Jackbean growth, with the precipitation slightly above the historical average.
Table 2 Climatic characteristics of the experimental period in the Instituto de Investigación de Cultivos de Raíces y Tubérculos Tropicales (INIVIT), in Santo Domingo, province of Villa Clara, Cuba, years 2012-2013. Cuadro 2. Características climáticas del período experimental en el Instituto de Investigación de Cultivos de Raíces y Tubérculos Tropicales (INIVIT), en Santo Domingo, provincia de Villa Clara, Cuba, años 2012-2013.
Five treatments were studied, consisting of simple inoculation of Jackbean seeds, with four strains of AMF, and a non-inoculated control. A completely randomized experimental design was used with three replicates, with a factorial arrangement of 5 x 2 and considering the year as a second factor.
The AMF species used belonged to the collection of the Instituto Nacional de Ciencias Agrícolas (INCA) and corresponded to the following: Funneliformis mosseae / INCAM-2 (Schüßler and Walker, 2011); Glomus cubense / INCAM-4 (Rodríguez et al., 2011); Claroideoglomus claroideum / INCAM-8 (Schüßler and Walker, 2011), and Rhizoglomus intraradices / INCAM-11 (Sieverding et al., 2014). The inoculants were produced in solid formulation according to Fernández et al. (2000), using Urochloa decumbens Stapf as a plant host and whose spore content ranged between 25 to 30 g-1 of the product.
Ten Jackbean seeds were sown in each microplot with a sowing distance of 0.5 m between rows and 0.2 m between plants. Mycorrhizal inoculation was carried out by covering the seeds at 8 % (inoculant / seed) and using 100 ml of water per kg of inoculant (Simó et al., 2016). Sixty days after germination, the content of mycorrhizal spores in the Jackbean rhizosphere, the percentage of total mycorrhizal colonization, as well as the aerial biomass of the plants, and the concentration of N, P and K were evaluated.
The methodologies used in the different determinations and evaluations were:
Number of spores in the soil
In each microplot spore extraction was performed following the procedure described by Herrera et al. (1995), they were counted using a stereoscope and the content was expressed as spores per 50 g of soil.
Total percentage of mycorrhizal colonization
To determine the total percentage of mycorrhizal colonization in C. ensiformis, each microplot was sampling and 200 mg of fine roots from each sample were used and dried in a thermostatically controlled oven, with forced ventilation at 70 ºC at constant mass. The roots were stained according to the methodology described by Rodríguez et al. (2015). The evaluation was made under a stereoscope. For the quantification the method of Giovanetti and Mosse interceptions (1980) was used.
Aerial dry biomass (ADB)
To determine the aerial dry biomass content, all the plants in each microplot were harvested, then the leaves and stems were separated. The dry mass (DM) in each sample was obtained from the percentage of dry mass of the aerial tissue, resulting from drying each sample in a thermostatically controlled stove, with forced ventilation at 70 ºC, until reaching a constant mass, the values were expressed in g m-2. The quantities of leaves and stems from each plot were added together and expressed in g per microplot.
Concentration of N, P and K
The concentration of N, P and K was obtained from tissue samples of dry sprouts, which were subjected to wet digestion with H2SO4 +Se (Kjeldahl method), the result was expressed in mg g-1. Nessler’s method was used to determine N, aminonaphthol sulfonic acid for P and flame photometry for K (Paneque et al., 2010).
Estimated extraction of N, P2O5 and K2O
The N, P2O5, and K2O extracted (g m-2) were determined as the concentrations of the elements (g kg-1) and the dry mass (g) in each organ studied, it was calculated by the following formula:
Extraction = MS x element concentration x gravimetric factor (1.2 for K2O and 2.29 for P2O5).
The quantities corresponding to leaves and stems in each plot were summed and expressed in g per microplot.
Statistical procedure
Once the assumptions of normality and homogeneity of variance of the data were verified, the results to determine the effectiveness of the mycorrhizal inoculation were processed as a completely randomized design with a factorial arrangement of 5 x 2. The factors and their respective levels were: AMF strains (four strains and one uninoculated control) and years (two). Tukey’s multiple range test (p≤0.05) was used as comparison criterion.
A regression analysis was performed to determinate the degree of mycorrhizal function obtained in each treatment, using the information obtained during the two years of work on the percentage of colonization, dry mass production, concentrations of N, P and K and number of mycorrhizal spores.
Results
Factorial analysis of all variables showed that there was no significant interaction between the main factors (AMF strains and years). The influence of AMF strains and years factors were significant. The results of the AMF strains factor are presented as they respond directly to the objectives of the work.
Mycorrhizal inoculation had a positive effect (p≤0.05) on the Jackbean biomass production, NPK contents and primary macronutrients extraction (Table 3) with a differentiated response (p≤0.05) between AMF strains. The plants inoculated with R. intraradices / INCAM-11 recorded the highest concentrations and extractions of biomass and nutrients with significant differences (p≤0,05) with respect to the rest of the treatments.
Table 3 Effect of the inoculation with four strains of arbuscular mycorrhizal fungi on biomass production, concentration, and extraction of macronutrient by C. ensiformis L. sixty days after sowing in each microplot. Calcaric Histosol soil of Santo Domingo, province of Villa Clara, Cuba, years 2012-2013. Tabla 3. Efecto de la inoculación con cuatro cepas de hongos micorrízicos arbusculares sobre la producción de biomasa, la concentración y la extracción de macronutrimentos por C. ensiformis L. a los sesenta días de la siembra en cada microparcela. Suelo Calcaric Histosol de Santo Domingo, provincia de Villa Clara, Cuba, años 2012-2013.
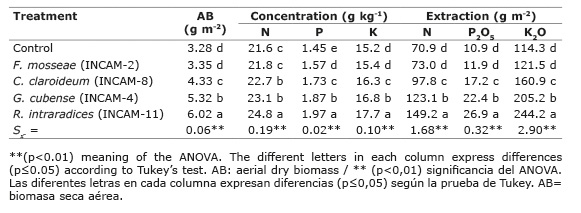
Of the remaining species, inoculation with G. cubense / INCAM-4 also had a positive effect with significant differences (p≤0.05) compared to the other species, except for the N content in which it did not differ from those obtained by the inoculation of G. claraideum / INCAM-8. The inoculation with the F. mosseae / INCAM-2 strain showed a much lower behavior than the other, with values similar to the control treatment.
Likewise, a significant (p≤0.05) and a varied effect of inoculation with AMF strains on mycorrhizal colonization percentages and spore content was found (Table 4), the highest values were always obtained with the inoculation of R. intraradices / INCAM-11 strain, with total mycorrhizal colonization percentages higher than 60 % and a 5.5-fold increase in spore content in the crop rhizosphere with respect to non-inoculated control. The rest of the strains presented a behavior similar to that obtained in the variables of biomass and macronutrient content.
Table 4 Inoculation effect with four strains of arbuscular mycorrhizal fungi on the total mycorrhizal colonization, and the spore content in the Jackbean rhizosphere at sixty days of sowing. Calcaric Histosol soil of Santo Domingo, province of Villa Clara, Cuba, years 2012-2013. Tabla 4. Efecto de la inoculación con cuatro cepas de hongos micorrízicos arbusculares en la colonización micorrízica total y el contenido de esporas en la rizosfera de Canavalia a los sesenta días de la siembra. Suelo Calcaric Histosol de Santo Domingo, provincia de Villa Clara, Cuba, años 2012-2013.
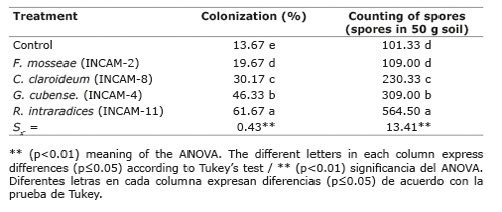
In general, the following behavior was obtained with respect to the response or efficacy of the AMF strains studied in each of the evaluated variables: R. intraradices / INCAM11 > G. cubense / INCAM4 > C. claroideum / INCAM8 > F. mosseae / INCAM2 = control.
Not only the inoculation with the R. intraradices / INCAM-11 strain reached the highest response in all evaluated variables, but high and significant determination coefficients were established, in the different treatments, between the percentages of total mycorrhizal colonization and the rest of the variables (Figure 1).
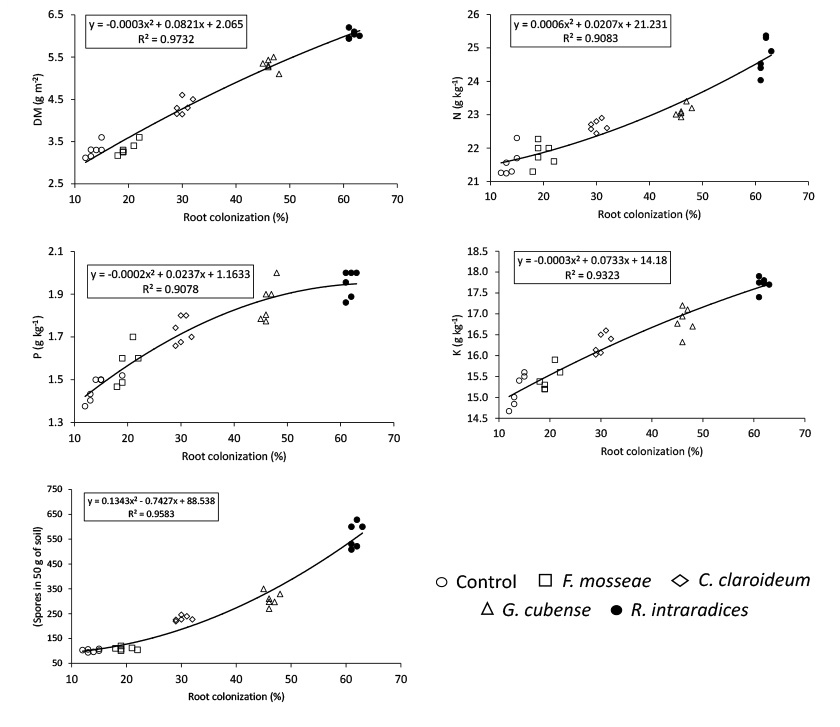
Figure 1 Relationships between the percentages of mycorrhizal colonization with the production of biomass (A); the contents of N (B), P (C), K (D); and with the number of mycorrhizal spores reproduced (E) in a Calcaric Histosol soil of Santo Domingo, province of Villa Clara, Cuba, years 2012-2013. Figura 1. Relaciones entre los porcentajes de colonización micorrízica con la producción de biomasa (A), los contenidos de N (B), P (C), K (D) y con el número de esporas de micorrizas reproducidas (E), en un suelo Calcaric Histosol de Santo Domingo, provincia de Villa Clara, Cuba, años 2012-2013.
The scatter plots and mathematical functions found for biomass (Figure 1A), P concentration (Figure 1C) and K concentration (Figure 1D), it seems to show a stabilization of the variables with the highest colonization; however, spores production (Figure 1E) and N content (Figure 1B) it seems to show a different behavior, since they did not suggest a stabilization, but an incremental increase with the highest values of mycorrhizal colonization.
Discussion
The positive effect of the inoculation with R. intraradices / INCAM-11 on the biomass and nutritional contents of Jackbean was explained by the establishment of an effective arbuscular mycorrhizal symbiosis and its beneficial effects on nutrient uptake (Willis et al., 2013; Yang et al., 2014) and thus on crop biomass production. The percentage of mycorrhizal colonization found in treatments with R. intraradices was around 60 %, a result that has been indicative of an efficient mycorrhizal functioning in most crops (Rivera et al., 2007) and in the Canavalia itself (Martín et al., 2015; García et al., 2017). Several Cuban authors have reported similar effects on mycorrhizal functioning, nutritional status and yields by inoculating different crops with efficient strains of AMF in different types of soils (Ruiz et al., 2012; González et al., 2015; Simó et al., 2016). Specifically, positive results have been reported for inoculation of the R. intraradices / INCAM-11 strain in different crops, such as grasses, bananas, Jackbean, cassava, rice, beans, among others, and types of soils and substrates, provided that the pH of these, in the depth of the soil in which the roots grow, is greater than seven (Rivera et al., 2015; González et al., 2016; João et al., 2016; Ruiz-Sánchez et al., 2016; Simó-González et al., 2017). The order of effectiveness of the different strains found in this work was also similar to that reported by previous authors in soils with light alkalinity conditions (pH between 7 and 8), suggesting that the strains respond differently and reproducibly to soil conditions.
Whit the inoculation of the F. mosseae / INCAM-2 strain there was no positive response, similar to that found in different crops in soils with pH≥7.4 (Göransson et al., 2008; González et al., 2016). Therefore, it appears that in the Calcaric Histosol soils whose soil reaction will always be higher than pH>7.5 the criteria obtained between the efficacy of the evaluated AMF strains and the soil pH are also met (Rivera et al., 2015), with R. intraradices / INCAM-11 being an efficient AMF strain in these soil conditions.
To control the distribution and consequent adaptation of the resident AMF species in agroecosystems, several authors (Göransson et al., 2008; Oehl et al., 2010) have pointed out the importance of soil and pH, these reports did not include inoculation of AMF strain and therefore could not evaluate the influence of pH on the efficacy of these strains.
The high values of mycorrhizal spores produced by the inoculation with R. intraradices / INCAM-11, are an indicator of effectiveness of the behavior of this strain under the conditions under study, they are also an indication of the ability of jackbean to reproduce mycorrhizal spores associated with the inoculation of the efficient strain. Similar increases in spore values have been found when inoculating Jackbean with efficient strains of AMF in different soil by Martín et al. (2009), Pentón et al. (2016), and Simó et al. (2016), which also reported a significant effect of permanence of the inoculant applied on the subsequent crop and related this permanence effect to the high reproduction of the spores, although the spores are not the only mycorrhizal propagules under these conditions (Willis et al., 2013).
The high determination coefficients obtained between the percentages of total mycorrhizal colonization and the rest of the variables indicated the importance of mycorrhizal functioning on the nutritional state of Jackbean and biomass production, which suggest that the differences in the effectiveness of the strains were a consequence of the different degrees of colonization reached with each one of them.
The high relationship between the colonization percentages and spore production could be due to the causal relationship between both variables, since the plants are first to be colonized, which causes the appearance of functioning mycorrhizal spores (Willis et al., 2013), but also indicates the importance of the spore number indicator as a criterion for mycorrhizal functioning.
Although some authors have reported that the inoculated strains cause greater growth and percentage of colonization, but these are not the ones that reproduce more spores (Chauhan et al., 2012); other authors (Furralzola et al., 2016) evaluated under natural conditions different species of grasses have not found a direct relationship between the percentages of colonization and spores, which is explained by the different reproductive capacity of the crops (Bever et al., 1996), as well as, possibly, in the different physiological state of them. Another effect occurs when comparing the effects of the inoculation of different generalist strains in the same crop. In several studies carried out in Cuba it has been found that the most effective strains have not only had a greater response in the biomass production and yield, with higher percentages of colonization, but also with more spores per g of soil (Martín et al., 2009; González, 2014; Martín et al., 2015; Gonzalez et al., 2016; Garcia et al., 2017). This is reinforced by the fact that the number of mycorrhizal spores is considered an adequate indicator to know the extent of the effect of permanence of the inoculant applied in Uroclhoa plantations (González, 2014).
Although the increases in spore production depends on the percentage of colonization, this is also a consequence of the number of mycorrhizal roots (Rivera et al., 2010; González, 2014). Both variables participate in spore production rate colonization increases. N content also behaved similarly, but in this case, can be explainable by the increase in biological nitrogen fixation (BNF), and therefore in N content, associated with the presence of an effective mycorrhizal symbiosis in legumes and a positive interaction between both symbiosis (Chalk et al., 2006; Yasmeen et al., 2012; Bulgarelli et al., 2017).
The different mycorrhizal colonization capacities found in the strains studied appear to be a consequence of the influence of the soil environment and presumably the pH (Rivera et al., 2015); however, there is no clarity as to the specific mechanism by which these factors influence the effectiveness of the strains.
Conclusions
Inoculation of Jackbean with the strain R. intraradices / INCAM-11 on Calcaric Histosol showed the greatest effects on biomass production, nutritional status and mycorrhizal function indicators, with higher results than when inoculate with G. cubense / INCAM-4, G. claroideum / INCAM-8 and F. mosseae / INCAM-2.
The high amounts of mycorrhizal spores associated with the inoculation of Canavalia with R. intraradices / INCAM-11 in this soil condition were indicative of the possibilities of using inoculated Jackbean as a way of subsequent mycorrhizal culture in this soil type.














