Introduction
The Hematopoietic Stem Cell Transplantation (HSCT) is a therapeutic procedure to treat onco / hematological diseases (Armitage,1994; Lange et al., 2006; Ribeiro et al., 1996; Ruiz-Argüelles et al., 2015). Prior the infusion of hematopoietic stem cells, an immunosuppressant regimen is necessary for death of bone marrow cells and preparation for reconstitution(Armitage, 1994; Bacigalupo et al.,2009). The most often conditioning regimens used for HSCT are reduced-intensity conditioning (RIC) and myeloablative (MA), and both can induce OM (Bacigalupo et al., 2009; Bardellini et al., 2013; Chaudhry et al., 2016; Turner et al.,2010). OM is an inflammation of gastrointestinal tract mucosa and its main cause is the chemotherapeutic conditioning prior to HSCT (Haverman et al., 2014; Stephen T Sonis, 2012; Stephen T. Sonis, 2012). OM can leave malnutrition, bacteremia, psychological alterations, increase of hospitalization days and, often, failure to finish immunosuppressant régimen (Haverman et al., 2014; Peterson et al., 2011; Vera-Llonch et al., 2007)OM is commonly evaluated by World Health Organization (WHO) Scale, which considers objective and subjective variables (WHO, 2017 ).
Although there are several clinical studies about OM, few studies evaluate OM daily during the immunosuppressant period. In order to establish strategies to improve the patient’s quality of life during the immunosuppressive phase it is required to evaluate the OM degree and its consequences throughout this period. Therefore, the aim ofthis study was to describe the incidence, severity of oral OM and related morbidities in individuals submitted to HSCT throughout the immunosuppression period.
Methods
Design
This is a longitudinal descriptive study conducted at the Bone Marrow Transplantation Unit of the Hospital de Clínicas of Federal University of Parana, Curitiba, Parana, Brazil, and was approved by the local Research Ethics Review Committee ( number: 1.431.294). Individuals’ recruitment was performed when they were at the beginning of the conditioning régimen prior to HSCT.
Subjects
Individuals with onco / hematological diseases, older than 14 years, both gender submitted to allogeneic HSCT were invited to participate of the study.
The participants agreed and signed the Free and Informed Consent Term / Informed Assent Term. Data were collected from March to October 2016.
Procedure
A trained dentist evaluated all participants. The presence and grade of OM, pain level, dysphagia, dysgeusia and xerostomia were assessed daily. The examination started two days before the infusión of hematopoietic stem cells and ended twenty days later. At the first day, all patients received oral hygiene and OM care instructions.
The OM was classified according to the WHO scale13 and visual analog scale (VAS) was used to measure pain level. The participants answered questions about dysphagia, dysgeusia and xerostomia. Clinical records were used to collect demographical data and to verify hematological disease, medications, patient’s diet and Body Mass Index (BMI).
Data analysis
Data were tabulated and organized using Software Statistical Package for the Social Sciences (SPSS) 20.0. The Shapiro Wilk tests were used to verify the normality of the sample. Variance Analysis (ANOVA) and post hoc of Tukey were used to compare the BMI with maximumdegree of OM. For statistic significant results was considered p <0.05.
Results
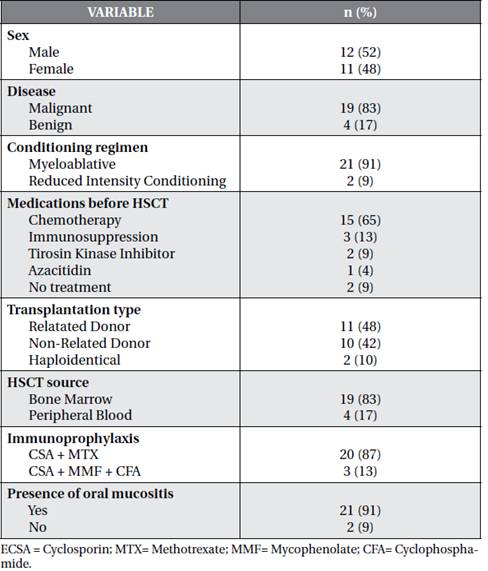
The initial sample was composed of 25 participants, of whom two were excluded. One had septic shock and the other one refused to participate. The median age was 31 years old (min. 14- max. 55) and male adults were predominant in the sample (52%). The majority of individuals had malignant diseases (83%) and MA conditioning (91%). Sixty five percent of the participants underwent chemotherapy prior the HSCT. Regarding the type of transplantation, 11 were related donor (48%), the main source used was the bone marrow (83%) and the most common immunoprophylaxis regimen was Ciclosporin + Methotrexate (Table 1).
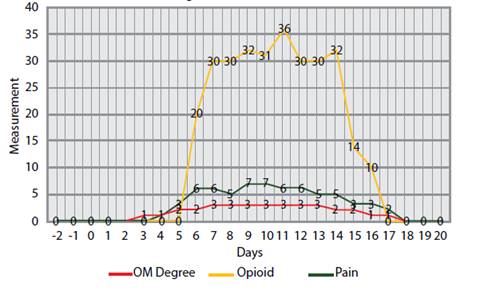
The incidence of OM was 91% (Table1). The median number of days with OM among individuals was 14 days (min. 0 - max. 26 days), while the median number of days with oral pain was 13 days (min. 0 -max. 25 days). On the other hand, the median OM grade, according to the WHO scale, was 3 (min. 0 - max. 4) and the maximum pain level was 9 (min. 0 - max. 10). Opioid medication was used for 11 days(min. 0- max. 21) and máximum dose used was 42 mg / day / EV(min. 0- max. 80mg / day / EV). The result of these variables can be found on chart 1.
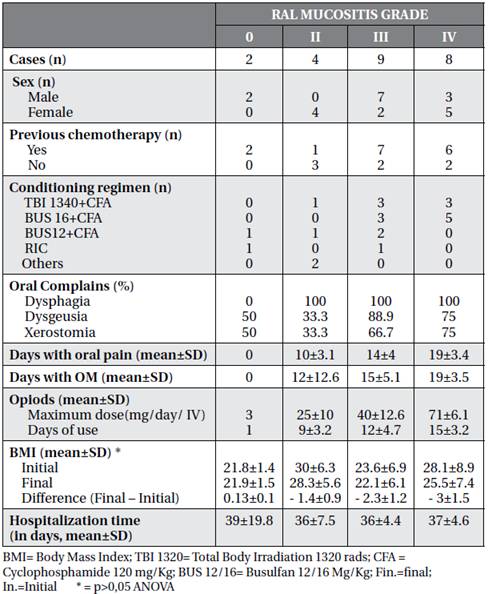
Based on the WHO scale, most of the participants had a máximum grade 3 (39%) of OM, individuals with this maximum degree of OM had mean of days of hospitalization similar to the mean of the other scores. Most individuals with grade 3 and 4 OM underwent chemotherapy prior to HSCT (Table 2).
The individuals who had the highest number of days with oral pain were also those who reached the maximum score of OM (19 days), stayed more days with OM, used opioid medication for more days (mean of 15 days ± 3.2) with higher doses (mean maximum 71 mg / Day EV ± 6.1). (Table 2).
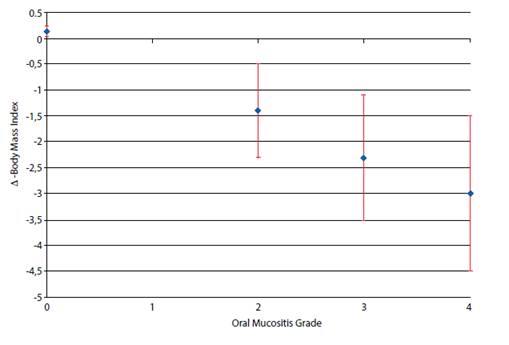
The conditioning that had the highest average number of individuals with grade 4 OM was Busulfan (BUS) 16 mg/kg + Cyclophosphamide (CFA) 120mg/kg. (Table 2). All individuals who had OM also had BMI reduction during the follow-up period. The mean loss of body mass from the beginning of hospitalization to the end was higher in individuals who reached grade 4 OM. However, there was no statistical significance (p>0.05 ANOVA). On the other hand, the two patients without OM had an increase in BMI. (Chart 2)
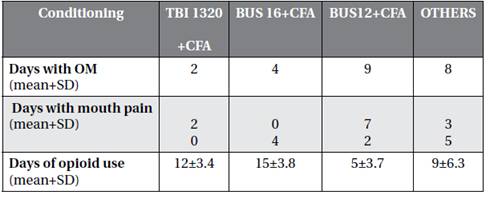
The individuals who had the highest mean number of days with OM and oral pain were those who had total body irradiation (TBI) 1320 rads + cyclophosphamide (CFA) 120 mg / kg as conditioning (Table 3).
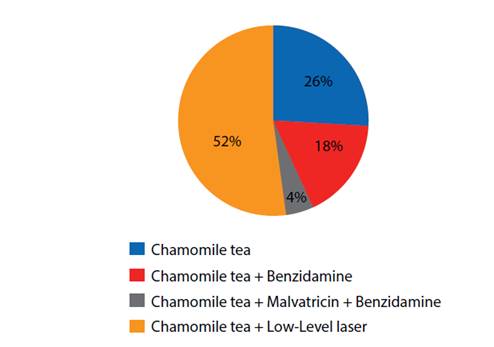
In addition to the opioid, which all patients with OM received, the most used treatment for OM was low-level laser associated with chamomile tea (52%)(Chart 3).
Topical treatments for OM were individualized according to the preferences and possibility of each subject, regarding the severity of OM, individual values, socioeconomic condition and above all the patient’s will.
Discussion
The present study proposed to evaluate the incidence and degree of OM, and disorders related to it in individuals submitted to allogeneic HSCT. The most important findings were the high incidence OM and the correlation between the OM severity degree, according to WHO scale, with the use of opioid medication and BMI. Individuals with higher OM scale values needed more opioid medication and lost more weight.
OM is a frequent condition in individuals submitted to HSCT, which affects their quality of life (Silva et al., 2015; Vera-Llonch et al., 2007).
In the present sample there were a great number of participants with leukemia, consequently, the conditioning regimens were mostly MA, which could explain the high incidence of OM (Eslami et al., 2016; Ramírez-amador et al., 2010; Small et al., 2007; Wojciechowicz et al., 2014).
The use of MTX as immunoprophylaxis may also have been a contributing factor to OM high incidence due to its high cytotoxic potential in cells with rapid multiplication. In addition, MTX is responsible for the delay of repair of the tissue damaged by chemotherapeutic conditioning (Ahmed et al., 2017; Cutler et al., 2005; Knoll et al., 2016; Matsukawa et al., 2016; Ramírez-amador et al., 2010; Small et al., 2007).
When compared to the total of days of hospitlization, a significant average of days with OM, oral pain and opioid medication were demonstrated.
This average changes when participants are segregated according to the maximum OM grade: Grade 4 individuals were affected with longer periods with OM, had more oral pain, more opioid using and with higher dosage than patients with lower scores.
There was a greater BMI loss in patients who reached grade 3 and 4 OM, although no statistical significance was detected (Table 2).
Regarding the conditioning regimen, individuals who used BUS 16 or TBI 1320 had a higher degree of OM (Table 1). Although most of the participants with grade 3 and 4 OM used BUS 16, who had a longer mean of days with OM and mouth pain were those that conditioned with TBI 1320, which suggests that such conditioning leads to prolonged toxicity. On the other hand, Chaudhry et al.(2016) performed a systematic review that evaluated the incidence and severity of OM in HSCT and found that there was a significant incidence of severe OM in both MA and RIC and those individuals who received TBI had more severe OM when compared to BUS. Nevertheless, it is important that individuals undergoing these types of conditioning receive extra oral.
Concerning the functional alterations the mouth, there was a high incidence of dysgeusia and xerostomia in patients with grade 3 and 4 OM (Table 2). Boer (Boer et al., 2009) already evaluated the taste alteration of patients submitted to HSCT and verified that the situation continues as a late complication. Furthermore, Laaksonen et al.(Laaksonen et al., 2011) studied longitudinally the saliva of patients for 24 months after HSCT, and also demonstrated some functional alteration in the mouth, concluding that hyposalivation is frequent and occurs more in MA conditioning and is reversible.
It is important to note that the use of opioid medication may often be associated with other painful episodes, not necessarily OM, so, it is important to evaluate other parameters, such as evaluation of mouth pain by VAS and the score of OM by the scale of WHO. Moreover,BMI should be used judiciously, especially in individuals with lean mass and / or water retention. In future studies it is suggested that body composition should be considered similarly to the study by Thomaz (ABESO, 2016; Thomaz et al., 2015).
One limitation of this study is the small number of participants. Further studies with higher simple size are suggested to evaluate the risk factors for OM and thus to delinéate a line of care for this condition. Although OM is a self-limiting condition and resolves with decreasing of chemotherapy toxicity and consequent bone marrow regeneration it is still a debilitating condition.
There fore, it is important to delimit its incidence, severity, risk factors and evolution time, for oral care can be implemented in the population at risk, in order to decrease the use of opioid medication and its side effects, improving the quality of life of this group.