Introduction
The heart rate (HR) elevation is related to increased mor tality and hospitalization for HF, its reduction improves the filling of the left ventricle, increases the myocardial oxygen supply and reduces its consumption, all of which is beneficial in patients with impaired left ventricular systolic function.1
Ivabradine is a selective inhibitor of the If currents in the pacemaker cells of the sinoatrial node, which, in humans, in duces to a heart rate reduction without modifying the inter ventricular or atrioventricular conduction or contractility.
It has been demonstrated that the use of Ivabradine in patients with heart failure in functional class II-IV, despite treatment, with a maximum dose or below it, with a beta-blocker or in those who do not tolerate it or when the use of beta-blockers is contraindicated, added to ACE inhibitors (ACEIs) or ARAs II or mineralocorticoid receptor antagonists (MRAs)3-5, with decreased systolic function (left ventricle (LV) ejection fraction (EF) less than 35%), in sinus rhythm and with a HR greater than 70 bpm reduced hospitalizations for HF and mortality for HF and, if this is greater than 75 bpm, it reduced cardiovascular mortality.3
The management of patients with advanced HF in mul tidisciplinary programs ensures a morbidity and mortality re duction with the highest levels of evidence.
Objective
To perform the first control of 26 patients treated with ivabradine in the PIC at HCB. A retrospective analysis was made with the first three years of all case material of patients with heart failure (HF) and reduced ejection fraction who were assisted in the PIC and received treatments recommen ded by the International Guidelines (angiotensin converting enzyme inhibitors II or angiotensin II receptor agonists, be ta-blockers and aldosterone antagonists) and maintained a heart rate greater than 70 bpm at rest in sinus rhythm, with the purpose of reducing it.
The baseline clinical data (blood pressure, heart rate, NYHA functional class and quality of life), natriuretic peptides and left ventricular ejection fraction by Doppler Echo cardiography at rest were then compared with the same variables in the follow up, because they are considered of prognostic importance.
For the analysis, anonymized records were used, identified by the values provided by the IT Department and the anonymized medical records review.
Methodology
A study with a population of 54 patients older than 40 years of age with HF who attended private outpatient care with cardiologists in the Greater Metropolitan Area of Costa Rica. For the data collection, a registration instrument was used in order to consider the relationship of all the relevant clinical data related to the prescribed dose, at the program entry after April 2013 and their follow up in outpatient care until August 30, 2016. The anonymized data were reviewed, and all patients who did not use ivabradine, after having sought the reason of non-use of the drug, were excluded.
The different dosage schedules used by physicians, who are cardiologists registered in the College of Physicians and Surgeons of Costa Rica, were identified. Modifications and periods for making the modification were assessed. Dosage changes over time were identified, as well as the impact on the rest of the pharmacotherapy. Because it is not a prospec tive study, six variables that were to be identified in all cases were validated in a baseline condition, even if it was before beginning the PIC. The variables were: heart rate, (systolic and diastolic) blood pressure, variations in the NYHA functional class, changes in the left ventricular ejection fraction (LVEF) measured by echocardiography with the Simpson method and natriuretic peptides BNP or Pro-BNP.
The data were analyzed and it was specified that there could be variables not found at the beginning or at the end of the evaluation period, but there should be at least 4 variables in order to consider if there was improvement in general, potentially attributable to the use of the ivabradine.
Every three to five months, the Kansas City Cardiom yopathy Questionnaire was administered to all PIC patients since April 2013; therefore, they were included in the general results of the baseline and end condition, with the purpose of considering if there was any impact on the quality of life.
Results
Of a total of 54 patients that were included in this analy sis, 26 were treated with ivabradine according to the Interna tional Guidelines for the treatment of HF.
The drugs considered as baseline are described as follows:
93% received angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II receptor antagonists (ARAs II) if they did not tolerate the first ones.
85% beta-blockers.
74% mineralocorticoid receptor antagonists.
The age range was from 46 to 95 years with an average of 78 years; 17 men and 9 women.
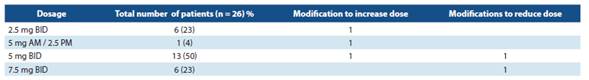
The dosage schedules shown in table 1 were identified.
In Costa Rica, ivabradine is only available with the name Procoralan® 5 mg and Procoralan 7.5 mg. Four (4) different dosage schedules were identified. The ones that are mostly used are those that correspond to 5 mg BID (twice a day) at the beginning (50% of the treated patients). Treatment was started in 69% of them following the international guidelines: in association with beta-blockers in those who were not pro perly controlled with an optimum dose of these drugs.
Six patients were identified with a dose of 7.5 mg BID with good tolerance. The dose was reduced in only 2 patients due to bradycardia.
Variable behavior
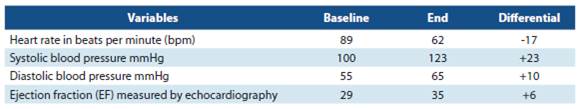
Table 2 includes the averages of heart rate in baseline condition and at the end of the control, blood pressure, left ventricular ejection fraction. All the patients were in sinus rhythm since their baseline condition. The results recorded at the end, occurred after receiving ivabradine during at least 2 months.
Regarding heart rate control, an average reduction of 17 bpm was achieved, which was considered an adequate response.4-5
The systolic blood pressure (BP) was more elevated than the diastolic BP. The LVEF measured by echocardiogra phy showed an improvement of 6% on average at the end of the analysis. In recent years, determining the natriuretic peptides (BNP) and their N- terminal fraction (NT-proBNP) values has shown to be of great support for the diagnosis of patients with suspected HF.6-8 In multiple studies, they have been qualified in different fields (primary care consultations, hospital emergency services) and have been shown to have a high negative predictive value. In this study, 16 patients had an average baseline BNP of 7 550 pg/ml, and 1 935 pg/ml on average at the end. Two patients had the NT-proBNP measu red once (reported values: 973 pg/ml at baseline condition. In another one, it was 3 266 pg/ml at baseline in one of the patients and 253 pg/ml at the end).
Variation behavior in the NYHA HF functional class
It was identified that 5 patients had improved from NYHA III at the baseline to NYHA I at the end, other 5 p presented improvement from NYHA III to NYHA class II, 5 patients did not improve the baseline NYHA functional status III and the NYHA III was maintained at the end of the study, and finally, in 3 patients there was deterioration and went from NYHA functional class II to NYHA III at the end.
The therapy duration and the survival condition within the analysis period were assessed.
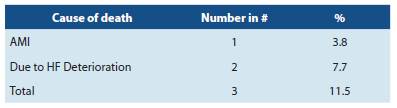
There was an 88.5% survival, 3 patients died as shown in detail in Table 3. All deaths occurred in the first year of follow up.
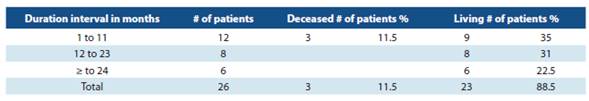
In the first three years of the program, patients that had not used ivabradine prior to the baseline condition were in cluded. Ivabradine was used by 53.5% of patients for more than one year. One patient received treatment with ivabradi ne for 33 months.
There was a patient in bradycardia with less than 50 bpm to whom ivabradine was interrupted. Serious adver se events were not related to ivabradine according to the treating physicians.
From the beginning of the PIC, 54 patients to date, 48% received ivabradine and most of them were on beta-blockers (85%). Overall, it was well tolerated. The most common side effects with ivabradine according to 9,10
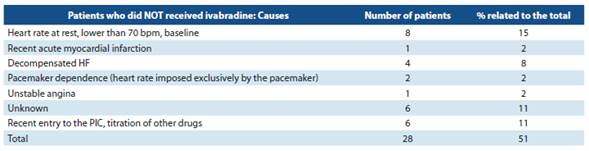
In table 5, the analysis of case material of patients who did not receive ivabradine (n = 28) is reported; of those, 16 patients had no indication. 10-12
Of the other 12 patients who did not use ivabradine, two causes were recent admission to the PIC, in titration stage of other drugs (n = 6), and the use of ivabradine is not mentio ned in the medical records of the rest (n= 6). With the Kansas City Cardiomyopathy Questionnaire (KCCQ-12), it was possi ble to determine an increase from 42 to 59 points, which was considered positive, and which may be related to the use of ivabradine, but not to mention other pharmacological and non-pharmacological interventions during the study which may also have contributed to this improvement. There was only one drug withdrawal registered due to significant brady cardia (less than 50 bpm).
In the PIC, of the 26 patients, all the variables were ob tained from 18 of them. Of the remaining group of patients (n = 8), four to five variables were found in baseline status, the same as at the end, which allowed the assessment of the improvement tendency.
Conclusions
Of the 26 patients that were studied, registered and treated with ivabradine in the PIC at HCB, most of them re gistered metric improvements identified as prognosis factors (heart rate, blood pressure, left ventricular ejection fraction, natriuretic peptides, functional class modification according to NYHA and quality of life). All the patients were assessed during an average period of 11 months. In patients with in complete data, at least four to five variables were found in the baseline literature are bradycardia, arterial hypotension, atrial fibrillation and status, the same as at the end. phosphe nes; only the first one was observed. The initial dose of iva bradine did not exceed 5 mg twice daily in patients younger than 75 years.
This registration, follow up and management of pa tients with HF through a PIC shows the importance that this type of programs has when using and prescribing drugs like ivabradine.