Introduction
Acianthera Scheidw. (Scheidweiler 1842) comprises ca. 300 species nested in the species-rich subtribe Pleurothallidinae (Brito et al. 2019, Chiron et al. 2012, Karremans et al. 2016, Pridgeon & Chase 2001, Rodrigues et al. 2017, Serna-Sánchez et al. 2021). Plants of Acianthera grow as epiphytes, lithophytes, or rarely as terrestrials in the cloud, wet or dry forests, ranging from Mexico and Antilles to Uruguay and Northern Argentina. The group exhibits its highest species richness in Brazil, where almost 120 species have been recorded (BFG 2015, 2018).
For decades, Acianthera was considered under a broad circumscription of Pleurothallis R.Br. (Brown 1813, Luer 1986). However, phylogenetic studies revealed Acianthera as a distinct and well supported monophyletic group (Karremans et al. 2016, Pérez-Escobar et al. 2017, Pridgeon et al. 2001, Serna-Sánchez et al. 2021) and, therefore, a broad consensus to recognize the genus (Damián et al. 2018, Karremans et al. 2016, Zambrano-Romero & Solano 2019).
Like many other genera in the Pleurothallidinae, Acianthera remains a poorly studied group in Colombia. About 30 species are recorded for the country (Bernal et al. 2019, Ortiz 2003, Ortiz et al. 2010). However, other neighboring Andean countries harbor more species than Colombia. For example, about 65 species are recorded in Ecuador (more than twice of Colombia), and new species are continuously revealed (Zambrano-Romero & Solano 2019). Therefore, the species diversity of Acianthera might be greater than presently recorded in Colombia (Bernal et al. 2019).
During orchid surveys conducted in the relict cloud forests of Valle del Cauca, Colombia in the western range of the Northern Andes cordillera, specimens of Acianthera were collected. They revealed morphological similarities with species recorded in Costa Rica, Panama, Colombia, and neighboring Andean countries such as A. geminicaulina (Ames) Pridgeon & M.W.Chase, A. decurrens (Poepp. & Endl.) Pridgeon & M.W.Chase, and A. erythrogramma (Luer & Carnevali) Luer (Luer 2004, Pridgeon & Chase 2001). However, comparisons of our field collected specimens with the available material for such taxa showed consistent breakdowns in the morphological continuum. Here, we propose it as a new species to science with the name of Acianthera hagsateri.
Materials and methods
Some flowers were preserved in liquid (70% ethanol, 20% water, 10% glycerol) before drying the plant. Dried material and digital specimens of A. decurrens, A. erythrogramma, and A. geminicaulina, available at CUVC, JBL, and VALLE and the herbarium databases of AMES, COL, MO, and W were consulted. Specialized literature and floristic catalogues were also consulted (Chiron & van den Berg 2012, Damián et al. 2018, Luer 2004). Morphological observations, dissections, and photographs of dried and spirit material of A. hagsateri and A. geminicaulina were carried out under a stereomicroscope provided by each herbarium. Acianthera hagsateri was described and illustrated by a composite line drawing from material collected in the field. The morphological description of A. hagsateri was based on five individuals gathered at the type locality. Distribution maps were generated using QGIS v.2.13. Distribution data of closely related species was obtained from the GBIF repository with the R-package SPOCC (Chamberlain 2017). Political division layers were downloaded from http://www.diva-gis.org/Data.
Taxonomic treatment
Acianthera hagsateri O.Pérez, L.Oses & Bogarín, sp. nov. (Fig. 1-2)
TYPE: Colombia. Valle del Cauca: Municipio de Dagua, Corregimiento de Queremal, junto al río San Juan, cerca del balneario, ca. 1400 m, 14 January 2012, Pérez O. & Parra E. 1488 (holotype: CUVC!).
Diagnosis: Acianthera hagsateri is similar to A. geminicaulina (Ames) Pridgeon & M.W.Chase, but differs in the small-sized plants, shorter ramicauls, smaller, succulent leaves, much shorter inflorescence born at the base of the leaves, dorsal sepal erect, linearoblong, petals mucronate with margins strongly serrated-lacerate, and by the entire, obovate lip.
Plant epiphytic, rhizomatous, small, to 5 cm tall. Rhizomes short, straight, the internodes up to 45 mm long. Roots filiform, greenish-white, sometimes stained with purple, up to 0.7-0.8 mm in diameter. Ramicauls short, stout, 4-10 mm long, erect, terete, slightly thickened toward the apex, covered by two scarious, tubular sheaths when young. Leaf 32 × 12-13 mm, sessile, ascendant, succulent, green stained with reddish-purple or sometimes completely reddish-purple, elliptical to ovate, acute, apex minutely 2-lobed, with a mucron between the lobes, margin entire, slightly convex on the adaxial surface, base cuneate. Inflorescence born from the apex of the stem, shorter than the leaf, racemose, 1to 3-flowered, ca. 5 mm long without flowers, including the pinkish-purple peduncle 1.5 mm long. Ovary 1.5 mm long, purple, minutely echinate, the trichomes conic, articled to a stout pedicel, 2 mm long, microscopically echinate. Floral bracts 3.5 mm long, obliquely funnel-shaped, long acute. Flowers relatively large in comparison with the plant size, to 2.4 cm in diameter, simultaneously-opening, bilabiate. Sepals pale purple, the nerves markedly pinkish-purple, membranous, minutely and sparsely verrucose on the abaxial surface, shortly hirsute, entire; the dorsal one 13.5 × 1.6 mm, linear-oblong, acute, shortly apiculate, slightly channeled, three-veined; the lateral ones connate into an ovate-lanceolate, obtuse, shortly apiculate synsepal, 11.2 × 4.5 mm long, convex near the apex, concave toward the base, chordate at the base, 6-veined. Petals 4.7 × 1.6 mm, translucent, membranaceous, slightly falcate, oblong-elliptical, acute, aristate, 3-veined, strongly serrate-lacerate along the margins, the margins, base, and nerves pinkish- purple. Lip 3.3 × 1.9 mm, similar in color to the sepals, fleshy, entire, verrucose, thickened towards the base, oblong, subacute, shortly apiculate, truncate at the base, three-veined; the disc with a small pair of marginal, thickened, slightly erect calli, extended from the base up to near the apex, channeled among them. Column 2.6 mm long, pinkish-purple at the base, green pale at the apex, stout, slightly curved at the middle part, ventrally channeled, thickened at the base, clinandrium denticulate, with a basal extension to form a very short foot. Stigmatic cavity ventral, suborbicular; rostellum ventral, helmet-like. Anther ventral, yellow. Pollinarium not seen. Capsule ellipsoid, 1 cm long, with persistent perianth.
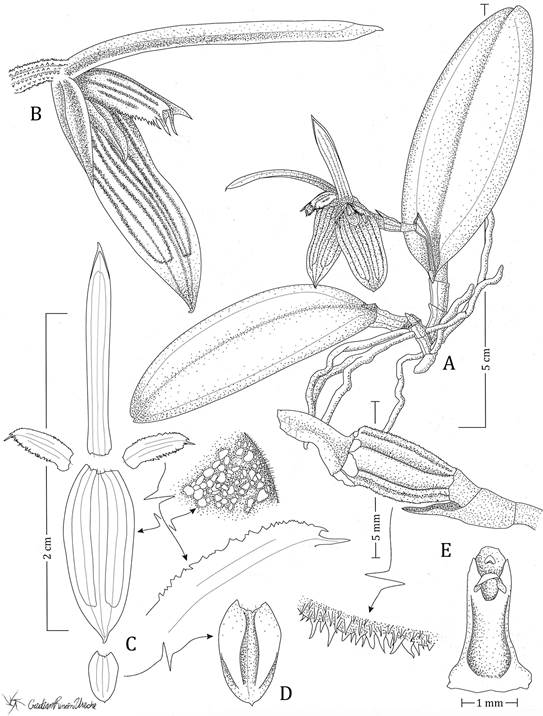
A. Plant habit.
B. Flower (3/4 view).
C. Floral dissection.
D. Lip
E. Column (side and ventral view)
Drawn by O. Pérez and C. Rincón-Useche from the holotype.
Figure 1 Illustration of Acianthera hagsateri.

A. Plant habit.
B. Flowers in situ.
C. Flower (side view).
D. Column, ovary, and pedicel.
Photographs by O. Pérez, from the holotype.
Figure 2 Acianthera hagsateri.
Additional specimens examined: Colombia. Valle del Cauca: Dagua, Corregimiento de Queremal, junto al camino que conduce al Cerro “Tokio”, ca. 1600 m, 29 May 2011, Pérez O. & Parra E. 1487 (VALLE!). Valle del Cauca: Dagua, Corregimiento de Queremal, carretera destapada que conduce al Cerro “Tokio”, ca.10 minutos en carro del casco urbano de El Queremal, ca. 1600 m, 18 March 2018, Pérez O., Galindo-T. & Zuluaga 1936 (CUVC!).
Distribution and ecology: So far, Acianthera hagsateri is known from the western slope of the Western Cordillera of the Andes, vicinity of Queremal, department of Valle del Cauca, Colombia (Fig. 3). The area is nested in the Northern Andes and Chocó biogeographic regions (Pérez-Escobar et al. 2019). It is known to be a center of orchid endemism (e.g. PérezEscobar et al. 2021a, Pérez-Escobar et al. 2022, Rodríguez & Blanco 2015), in particular of Pleurothallidinae (e.g. Farfán et al. 2003, Kolanowska & Pérez-Escobar 2012, Peláez et al. 2009, Pérez-Escobar et al. 2013). It grows as an epiphyte on isolated trees and remnant cloud forests at 1600 m elevation, where it appears to be abundant. It flowers throughout the year, and developing fruits were observed in June.
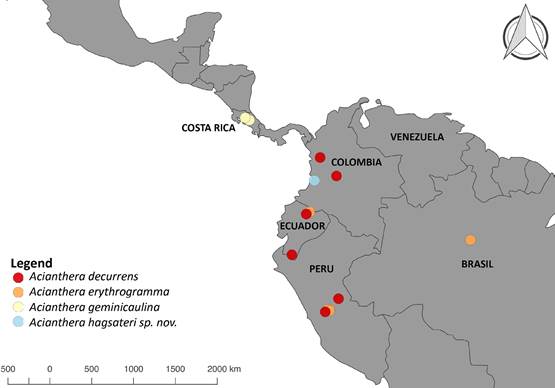
Map by O.A. Pérez-Escobar.
Figure 3 Distribution map of Acianthera hagsateri, A. decurrens (based on Poeppig 1604 W!, Luer et al. 19691 MO, Betancur et al. 7018 COL!, Croat 69890 MO), A. geminicaulina (based on Karremans 2990 JBL!, Karremans 5209 JBL!, Karremans 3205 JBL!, Karremans 5210 JBL!) and A. erythrogramma (based on Luer et al. 11766 MO!, Berlin 1605 MO).
One population was reported growing prolifically on Myrsine sp. (Myrsinaceae), beside a river.
Eponymy: Named after Eric Hágsater Gartenberg, chief Director of the AMO Herbarium in Mexico. Eric has enormously contributed over the past 50 years to the taxonomy and systematics of the genus Epidendrum and the Mexican orchid flora. His monographic work on the genus has led to the thorough documentation of the morphological variation, distribution and ecology of thousands of orchid species in the American tropics, while promoting the training of dozens of young Latin American botanists.
Discussion.
Acianthera hagsateri resembles A. geminicaulina, a species described as Pleurothallis geminicaulina Ames based on a plant collected by Charles H. Lankester at La Unión, Turrialba, Costa Rica; then it was recorded in, Panama, Colombia, and Ecuador (Luer 2004). While studying the morphological variation of A. geminicaulina in Costa Rica from four localities, including the type locality, we observed that Colombian specimens differ from those of Costa Rica. Acianthera hagsateri differs from A. geminicaulina mainly in the shorter ramicauls < 1 cm long (vs. 3-8 cm), shorter inflorescences < 5 mm (vs. of 10-15 mm), the pale purple sepals with markedly pinkish-purple veins up to the apex (vs. purple or reddish without prominent purple veins), the erect dorsal sepal (vs. suberect or patent), the petals with three markedly pinkish-purple veins (vs. only the midvein purple-reddish) and the oblong, verrucose lip (instead of elliptical-oblong, smooth). The morphological variation of the lip in A. geminicaulina and A. hagsateri is shown in Fig. 4. All morphological differences among the new taxon and its closest relatives are summarized in Table 1.

A. Karremans 2990, Costa Rica. Cartago: Santa Cruz, 9'57''8''N 83'43''3''W, elevation: 1350 m JBL!.
B. Karremans 5209, Costa Rica. Alajuela, 10'16'N 84'09'W, elevation: 900 m JBL!.
C. Karremans 3205, Costa Rica. Cartago: Turrialba, 10'00'06''3''N 83'41'47''5''W, elevation: 1470 m JBL!
D. Karremans 5210, Costa Rica. Alajuela, 10'16'N 84'09'W, elevation: 900 m JBL!.
E. Pérez O. & Zuluaga 1936 (CUVC!). Scale bar= 3 mm.
Photos by L. Oses.
Figure 4 Morphological diversity of the lip in individuals of A. geminicaulina (A-D) and A. hagsateri (E)
Acianthera hagsateri is further similar to A. decurrens, a species from Ecuador and Peru, but differs in its smaller habit, up to 4 cm (vs. 20-45), shorter leaves up to 32 mm long not decurrent along the apex stem (vs. 10-15 cm long, decurrent along the apex), shorter ramicauls 4-10 mm long (vs. 10-30 cm long), shorter inflorescence < 5 mm (vs. 40-50 mm), petals elliptical, aristate at the apex, not callose (vs. obovate, long acuminate, bicallose at the mid-part) and the obovate lip, with margins entire, bicallose near the base (instead of elliptical with margins minutely serrate below the middle, bicallose above the middle). Acianthera hagsateri also resembles A. erythrogramma, which is restricted to the Amazonian lowland forests of Brazil, Ecuador and Peru and has larger (6-8 cm vs. 0.4-1.0 cm), triquetrous ramicauls (vs. rounded, cylindric), larger, broadly elliptical to suborbicular leaves and chordate at the base (vs. elliptical to ovate, rounded at the base), fasciculate-like inflorescences (vs. racemose), yellow, glabrous sepals (vs. pale purple and minutely verrucose), and a larger elliptical lip (5.5 mm long) with denticulate margins (vs. 3.3 mm and entire margins).
The Acianthera clade comprises the genera Acianthera, Antilla Luer, Brenesia Schltr., and Kraenzlinella Kuntze. These groups have also been treated at subgeneric level under a broad concept of Acianthera (Karremans et al. 2016, Pérez-Escobar et al. 2017, Pridgeon et al. 2001, Serna-Sánchez et al. 2021). Among them, the most diverse group is Acianthera subgenus Acianthera concentrating almost 90% of the species of the clade. Several generic concepts have been proposed within this group, but none is supported yet by molecular data. The taxon sampling and DNA sequencing of Acianthera s.l. is still deficient (e.g., Pérez-Escobar et al. 2021b). Thus, systematic interpretations in Acianthera and the Pleurothallidinae have been based on studies that included a limited number of molecular markers leading to poorly supported and unresolved phylogenies (Bogarín et al. 2018, 2019, Karremans et al. 2016, Rodrigues et al. 2017). Morphologically, A. hagsateri belongs to Acianthera subg. Acianthera.
Table 1 Main morphological differences among Acianthera hagsateri, A. decurrens, A. erythrogramma, and A. geminicaulina.
| Character | A. hagsateri | A. decurrens | A. erythrogramma | A. geminicaulina |
| Plant size | up to 4 cm | up to 32 cm | up to 12 cm | up to 16 cm |
| Ramicaul | Terete, 0.44-1.00 cm long | Terete, 10-30 cm long | Triquetrous, 6-8 cm long | Terete, 3-8 cm long |
| Leaves | Elliptical to ovate, 3.2 × 1.2-1.3 cm | Elliptical, decurrent, 10-15 × 4-6 cm | Broadly elliptical to suborbiculate, 3.5-4.5 × 35-4.5 cm | Oblong-elliptic, 3-8 × 1.5-2.5 cm |
| Inflorescence | 5 mm long | 40-50 mm long | ca. 10 mm long | ca. 10-15 mm long |
| Petals | elliptical, margin strongly serrate-lacerate, translucent with the base, margins, and nerves purple | obovate, entire, green | Obovate, serrate, translucent | Elliptical-obovate, serrate, translucent with a purple midvein |
| Lip | Oblong, obtuse, entire, verrucose. Shortly apiculate with a pair of calli near the base and two parallel keels above the middle | Elliptical, slightly serrate, smooth. Disc depressed, surrounded by parallel calli above the middle, and with a callus below the middle reaching the base (Luer 2004) | Elliptical, obtuse, denticulate, with a pair of verrucose calli within the margins in the mid-part (Luer & Carnevali 1993). | Elliptical-oblong, obtuse, entire, smooth. Shortly apiculate with a pair of calli near the base and two parallel keels above the middle (Luer 2004). |












 uBio
uBio 


