Introduction
The orchid family is one of the most species rich families of seed plants. It includes about 880 genera with more than 25,000 species in the world (Cribb et al. 2003). Due to mycorrhizal specificity (McCormick & Jacquemyn 2014), pollinator specialization (Cozzolino & Widmer 2005) and limited germination rates (Dearnaley et al. 2012), most orchids are extremely susceptible to habitat disturbance as compared to other plants (Cozzolino & Widmer 2005, Jacquemyn et al. 2007). Orchids frequently occur in very specific habitats (Wotavova et al. 2004). They are particularly vulnerable to climate and land-cover changes due to their narrow ecological preferences. A high proportion of species have declined in abundance and are considered to be rare, threatened, or endangered, primarily as a result of habitat loss (Kull & Hutchings 2006, Pillon & Chase 2007). Patterns of orchid richness are regulated by habitat size and elevation range at large scales (Acharya et al. 2011), while by light availability, soil moisture, canopy height and area (especially for the epiphytic orchids) at fine scales (McCormick & Jacquemyn 2014).
About 130 orchid species belonging to 42 genera are documented in the Russian Federation (Varlygina 2011). Due to the increase of anthropogenic influence and natural features of orchid species many of these plants are endangered and 65 species were included in the Red Data Book of the Russian Federation (Bardunov & Novikov 2008). There are 39 orchid species belonging to 20 genera in Central Russia (Averyanov 2014). Of these, 19 species were included in the Red Data Book of Russian Federation (Bardunov & Novikov 2008).
Cypripedium calceolus L. is one of the most attractive terrestrial representatives of Orchidaceae in Eurasia. Its distribution ranges from Great Britain and Scandinavia across Northern and Central Europe to North-Eastern Spain, Northern Italy, and from Western Europe through southern Siberia to Rebun Island (Bardunov & Novikov 2008, Cribb 1997, Hultén & Fries 1986, Kull 1999, Vakhrameeva et al. 2014). Although, C. calceolus is included in the Global IUCN Red List with category Least Concern (LC), this is one of the most endangered orchid species in Europe (Kull 1999). In different regions of Eurasia, C. calceolus has been estimated as Near Threatened (Bilz 2011, Turis et al. 2014), Vulnerable (Rassi et al. 2001), Endangered (Witkowski et al. 2003), and Critically Endangered (Blinova & Uotila 2011, Khapugin et al. 2017a, Khapugin et al. 2017c). International studies on C. calceolus related to its genetic diversity (Brzosko et al. 2009, Brzosko et al. 2011, Fay et al. 2009, Kļaviņa et al. 2014, Minasiewicz & Znaniecka 2014), population ecology and biology (Blinova 2002, Brzosko 2002, Davison et al. 2013, Fardeeva et al. 2010, Gajewski & Marcisz 2014, Khapugin et al. 2014, Korczyński & Krasicka-Korczyńska 2014, García et al. 2002, García et al. 2010, Gorchakovskii & Igosheva 2003, Kirillova 2015, Kull 1998, Nicolè et al. 2005, Puchnina 2017, Stetsuk 2013, Zheleznaya 2015), impacts of environment conditions and stress-factors on the species (Blinova 2002, 2012, Czerepko et al. 2014, Kirillova 2016, Kirillova et al. 2012, Puchnina 2017, Shefferson et al. 2012), pollination (Antonelli et al. 2009, Tremblay 1994), as well as the list of publications with new records of this threatened species is continuously enlarging (e.g. Balázs et al. 2016, Matysek et al. 2014, Piwowarski 2013, Puchnina et al. 2015, Randić et al. 2013). However, there is a lack of data on the status of C. calceolus populations in many parts of its natural distribution range.
Cypripedium calceolus is included in the Red Data Book of the Russian Federation (Bardunov & Novikov 2008). It is known from 20 populations in the Republic of Mordovia (Central Russia). However, 14 sites are located outside of the existing protected areas network (Khapugin et al. 2017b). Moreover, actual protection of C. calceolus is provided only for three populations in the Mordovia State Nature Reserve. Thus, the status of other populations still needs to be monitored in order to understand what factors could contribute to deplete the C. calceolus populations there.
Cypripedium calceolus is an emblematic plant species in the Republic of Mordovia. Its picture has been portrayed on the cover page of 1st edition of the Red Data Book of the Republic of Mordovia (plants, fungi, lichens) (Silaeva 2003). Recently, the regional IUCN status of this species was estimated as Critically Endangered (Khapugin et al. 2017a,c). However, data on individual populations are fragmented. Continuous six-year studies have been carried out only in the Mordovia State Nature Reserve. Two local C. calceolus populations at the boundary of the National Park “Smolny” have been studied for three years. In addition, fragmentary data of different years on two more C. calceolus populations in Bolshie Berezniki district of Mordovia, are available. But to date these fragmented data were not generalized.
The aim of the present study was to study population structure, biology and ecology of C. calceolus in Central Russia, emphasizing its populations in two federal Protected Areas in the Republic of Mordovia: Mordovia State Nature Reserve and National Park “Smolny”. The following questions were addressed: (I) What is the status of C. calceolus populations protected by Mordovia State Nature Reserve and National Park “Smolny”? (II) Are there differences between the C. calceolus populations studied?
Materials and methods
The Republic of Mordovia is located on the border of the forest and forest-steppe zones in Central Russia. The eastern part of the Republic of Mordovia covers the north-west of the Volga Upland, and its western part is located on the west of the Oka-Don lowland. High habitat diversity is observed, coniferous and mixed forests are distributed in the west and north-west, broad-leaved forests are distributed in the central and eastern parts, and foreststeppe landscapes dominate in the east and south-east (Yamashkin 1998, 2012).
Cypripedium calceolus is a long-lived perennial that may survive more than 30 years (Kull 1988). This is a geophyte with a horizontal rhizome growing up to 10 cm underground. Stems are 20-60 cm in height with 3-5 elliptical to ovate-oblong leaves, which are 11-17 cm long and 5.5-8 cm wide. It produces one or two (rarely three) large flowers, with a purple-brown perianth and yellow shoe-shaped labellum. The fruit is a capsule (3 cm long and about 0.9 cm in diameter) with 6,000-17,000 seeds (Denisova & Vakhrameeva 1978, Tatarenko 1996, Kull 1999).
Most well-studied populations of C.calceolus in the Republic of Mordovia are located in the Mordovia State Nature Reserve (MR), National Park “Smolny” (NP) and in the vicinity of the biological station of the Mordovia State University (BS). Of the three populations in MR, only one is still known. One population has disappeared after a wildfire in 2010, while a second population is very small (up to 10 individuals) and does not appear every year. Results of populationbased studies in the Mordovia State Nature Reserve (MR) and in National Park “Smolny” (NP) were compared. Data for the populations’ stage structure and floristic composition in the vicinity of the biological station of the Mordovia State University (BS1: about 750 m2, 54.188541 N, 46.172314 E; BS2: about 350 m2, 54.183617 N, 46.175930 E) were available from 2012 and 2015. The field investigations were carried out for the populations in MR (about 1000 m2, 54.716110 N, 43.205170 E) in 2011-2016, for population NPt near Tashkino village in 2014-2016 (about 100 m2, 54.736807 N, 45.505268 E), and for the population NPk near Kamchatka village during 2012, 2014 and 2016 (about 350 m2, 54.726835 N, 45.457555 E).
To get a complete overview of the species’ situation, abundance of individuals and morphometric traits of plants of Cypripedium calceolus populations were studied on established square (1×1 m) plots. In total, three (NPt) and six (NPk, MR) square plots were established in studied habitats. The composition of the accompanying flora was recorded on square plots (15×15 m) with respect to each location for further interpretation. The abundance of each species has been estimated in accordance with the scale of BraunBlanquet (1964). The nomenclature is in accordance with The Plant List (2013) and Euro+Med Plantbase (Euro+Med 2006+). Jaccard’s similarity index was calculated (Jaccard 1901).
Based on the species composition of flora within each C. calceolus location, the mean environment indicator values (EIVs) for each studied habitat was calculated. For this purpose, Tsyganov’s (1983) ecological scale was used, where EIVs are arranged as intervals. Therefore, for each plant species the range of its existence in relation to a concrete factor, for instance, soil nitrogen, moisture etc., can be defined. Values could be evaluated in conventional units covering the total factor range from the minimum up to the maximum in relation to concrete species. Mean values were calculated using an algorithm suggested by Buzuk & Sozinov (2009). Six environmental factors were examined: shadiness (L), temperature (T), continentality (C), moisture (M), pH (R), and soil nitrogen (N). In the framework of our study each above-ground shoot was the accounting unit being conditionally treated as an individual. Based on revealed morphometrical data (height of shoot, number of leaves and leaf veins, presence/absence of flowers), individuals of C. calceolus were divided into four ontogenetic stages: juvenile (j), immature (im), mature vegetative (v) (non-flowering adults) and generative (g) (flowering adults) (Denisova & Vakhrameeva 1978, Vakhrameeva & Tatarenko 1998). The group of mature vegetative individuals comprised both virginal and generative plants which failed to form generative shoots and, hence, were in the vegetative state in the study year. Depending on the ratio between these age groups, three types of the stage spectra of populations were distinguished according to Gorchakovskii & Igosheva (2003): vegetatively-oriented (with the prevalence of juvenile, immature, or mature vegetative individuals), generatively-oriented (with the prevalence of generative individuals), and bimodal (with two peaks - one accounted for by vegetative and the other, by generative individuals). Stage spectra of C. calceolus populations at the Republic of Mordovia were compared with results of previous demographic studies carried out in Spain, Estonia, Poland, Central and Northern Russia and Siberia (Fig. 1).
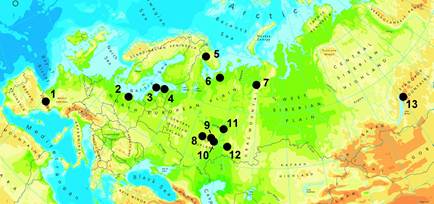
Figure 1 Geographical location of some Cypripedium calceolus populations within its range. Locations: 1. Sallent, Pineta, Tormosa, Ordesa; 2. Lake Kwiecko; 3. Muhu I, Muhu II, Hiiumaa, Puhtu; 4. Ussisoo, Tooma, Õisu I; 5. Apatity; 6. Pinega 1, Pinega 2; 7. “Yugyd va” 1-7; 8. Mordovia State Nature Reserve (MR); 9. neighbourhoods of villages Tashkino (NPt) and Kamchatka (NPk) near National Park “Smolny”; 10. neighbourhood of the biostation of the Mordovia State University (BS1, BS2); 11. Republic of Tatarstan; 12. Buzuluksky Bor; 13. Barguzinsky Reserve. Detail data on each location - in Table 3. Map with modifications from http://www.eea.europa.eu/legal/copyright
In 2014, the C. calceolus population in the Mordovia State Nature Reserve was suggested as the most sustainable in the region. The total number of generative individuals, number of plants with two flowers and the fruit set were investigated in the same population in our study. The sustainability of the population was estimated on the basis of successful reproduction of plants (e.g. Bizoux et al. 2011, Hegazy et al. 2010, Oostermeijer et al. 2003).
Statistical analyses were carried out using PAST 3.15 (Hammer et al. 2001). The ordination techniques, using the principal component analysis (PCA), defined the major gradients in the spatial arrangement of studied habitats of the analyzed dataset. For ecological interpretation of the ordination axes, average environment indicator values were calculated for established plots and were plotted onto the PCA ordination diagram as supplementary environmental data.
Results
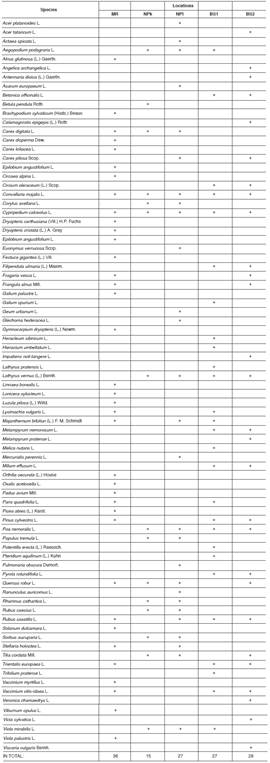
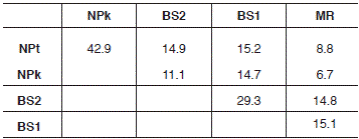
The composition of flora accompanying C. calceolus was documented in all five locations studied. The list includes 80 species of vascular plants from 69 genera and 37 families (Table 1). Of these, 36 species from 32 genera and 22 families in MR, 15 species from 15 genera and 13 families in NPk, 27 species from 25 genera and 21 families in NPt, 27 species from 26 genera and 16 families in BS1 and 28 species from 27 genera and 20 families in BS2. The Jaccard’s similarity index amongst accompanying flora varied from 6.7% (amongst MR and NPk) to 42.9% (amongst NPt and NPk) (Table 2).
Cypripedium calceolus inhabited shade or semishade forest communities, where only Convallaria majalis L. was an obligatory component. Four species (Lathyrus vernus (L.) Bernh., Poa nemoralis L., Quercus robur L., Rubus saxatilis L.), were registered in four of the five habitats; and seven species (Aegopodium podagraria L., Carex digitata L., Maianthemum bifolium (L.) F.M. Schmidt, Tilia cordata Mill., Trientalis europaea L., Vaccinium vitis-idaea L., Viola mirabilis L.) were found in three of the five studied sites. The spatial arrangement of studied locations demonstrated that the MR site clearly separated from all other habitats on the base of higher soil moisture and lower values of soil pH and light level. Both habitats from the vicinity of Mordovia University biostation (BS1, BS2) are closely located at the PCA-ordination diagram due to their close geographical position. Consequently, these habitats have similar environmental conditions (Fig. 2). These three locations (MR, BS1, BS2) are well separated from the NPk and NPt sites on the basis of low values of soil nitrogen, soil pH and light level. The NPk and NPt sites are closely located at the PCA-ordination diagram. The same picture is obtained for BS1 and BS2 sites. Revealed data obtained using phytoindication method (Fig. 1) are very similar to the results found using Jaccard’s similarity index (Table 2).
Table 1 List of flora accompanying Cypripedium calceolus in the Republic of Mordovia (Central Russia).
The stage spectrum in C. calceolus populations in Mordovia was presented by four ontogenetic stages (Fig. 3). Due to the poor and random demographic data from populations in both the BS1 and BS2 sites, these have been excluded from analysis. Vegetative (juvenile + immature + mature vegetative) individuals dominated in the stage structure of C. calceolus populations during the study period (Fig. 2). Therefore, these populations could be considered as vegetatively-oriented. However, in certain years some fluctuations were recorded, allowing interpreting these populations as moderate or even generatively-oriented: NPk population in 2012, NPt population in 2014 or MR population in 2016. In contrast, fragmentary demographic data on both BS1 and BS2 populations showed dominance or equality of generative individuals in stage spectra (Khapugin et al. 2014).
Table 2 Compositional similarity (Jaccard’s index, 100 × J) of the accompanying flora in five locations with Cypripedium calceolus in the Republic of Mordovia (Central Russia).
We estimated the reproductive ability of C. calceolus in the most sustainable population in Mordovia based on a number of two-flowered generative individuals and fruit set in the population (Fig. 4). We found that the percentage of plants with two flowers varied from 0.0% in 2015 to 16.7% in 2016. And there was no correlation of this parameter neither with the number of generative plants in a population nor with the percentage of two-flowered individuals there. Similarly, we didn’t find a correlation of these parameters with values of fruit set in the MR population during the study period. It varied from 27.8% in 2016 to 46.7% in 2012.
Discussion
The accompanying flora in the studied sites was similar. In accordance with both PCA-analysis and Jaccard’s similarity index, the most significant similarity was found for NPt vs. NPk and BS1 vs. BS2 respectively. These results can be explained by similar environment conditions in these habitats. At the same time, three types of plant communities were defined, demonstrating the ecological variability of C. calceolus due to its inhabitation in boreal (MR), steppefied nemoral (NPt, NPk) and mixed (BS1, BS2) forest communities. Unfortunately, there is no record of C. calceolus in grassland habitats in the Republic of Mordovia. Similar results shown using the Jaccard similarity index and phytoindication methods allow us to propose their conjoint use for other plant species as it was tested earlier (e.g. Couto et al. 2016, Khapugin et al. 2016, Khapugin 2017, Michálková 2007). Cypripedium calceolus populations protected by Mordovia State Nature Reserve and National Park “Smolny” could be considered as vegetatively-oriented. However, the portion of generative individuals was significant over the whole study period varying from 31.3% in NPk to 39.2% in MR. Generative plants had dominated in different time periods of the study in these habitats, even though there are some populations characterized by the dominance of generative plants in the region. On the basis of previous studies (Khapugin et al. 2014), with additions (Table 3), both populations BS1 and BS2 had generatively-oriented stage spectra. The total abundance of individuals in a population did not affect its stage spectrum structure. For instance, a general low decrease of total abundance in MR population led to the dominance of generative individuals in 2016, while the general increase of individuals’ number in the NPk population led to the dominance of vegetative individuals in the same year. These results are consistent with data of Kull (2003) who suggested that neither short-term nor long-term studies of C. calceolus populations could show any clear trends in their stage spectrum structure due to the effect of annual fluctuations in abundance of individuals. The author also proposed that a large percentage of juvenile plants could be considered as a good indicator of a vital population, and that the population with few juvenile plants may persist, as long as the habitat conditions are suitable if vegetative reproduction in the population is significant and a number of generative individuals is not too low (Kull 2003). Most C. calceolus populations were estimated as vegetatively-oriented in different locations within its range (Table 3). Of all compiled data, the populations located at open or semi-open habitats are characterized by dominance or equality of generative individuals in stage spectra. For instance, these are semi-shade and grassland habitats in locations Sallent and Pineta in Spain Pyrenees (García et al. 2010), the edge of the spruce-larch forest in the National Park “Yugyd va” (Kirillova 2015), populations BS1 and BS2 in Mordovia (Khapugin et al. 2014, with additions). Light level may perhaps be one of the factors determining the stage spectrum of C. calceolus populations.
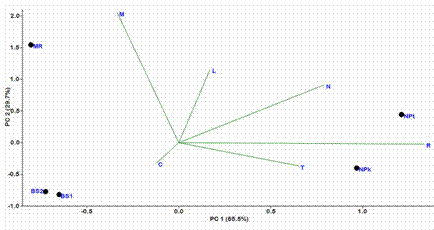
Figure 2 Principal component analysis (PCA) ordination diagram of habitats with Cypripedium calceolus in the Republic of Mordovia (Central Russia) based on mean environmental indicator values. Designations: L. shadiness, T. temperature, C. continentality, M. soil moisture, R. soil pH, N. soil nitrogen. To reveal ecological gradients the mean environmental indicator values were plotted onto PCA-ordination diagram as supplementary environmental variables.
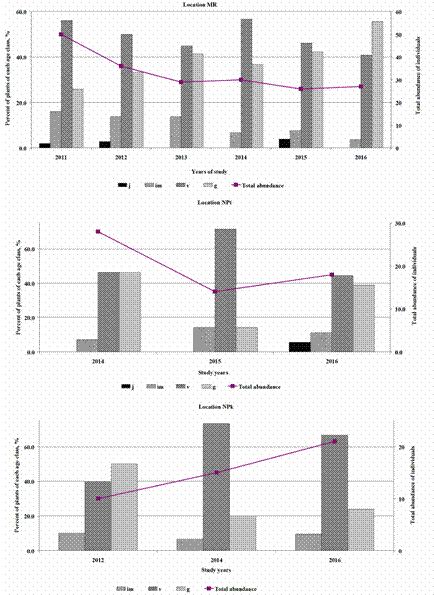
Figure 3 Structure of stage spectra in Cypripedium calceolus populations (locations MR, NPk, NPt) in the Republic of Mordovia (Central Russia). Ontogenetic stages of individuals: juvenile (j), immature (im), mature vegetative (v), generative (g).
Among all studied populations in Mordovia, only the MR population could be considered as such a population with low but persisting number of juveniles, active vegetative reproduction and the appropriate number of generative individuals. Thus, this population may be considered as the most sustainable. However, an increase of red squirrel (Sciurus vulgaris Linnaeus, 1758) abundance in MR habitat in recent years led to increasing of young Picea abies abundance in the shrub layer. Thus, conditions of special protection regime could lead to the decrease of C. calceolus population vitality (Kull 2003). Hence, a further decline in
Table 3 Lady’s Slipper (Cypripedium calceolus) locations and habitats where age spectra were estimated
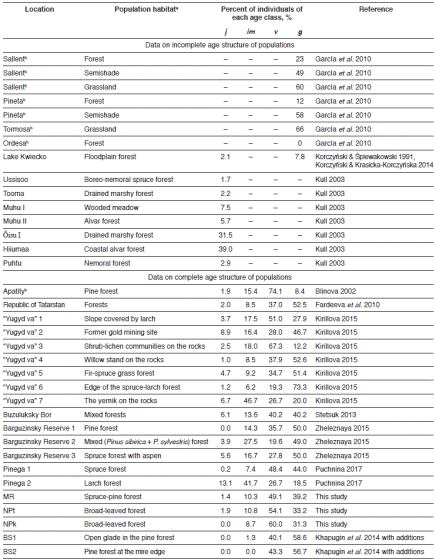
a We use the names of habitats referred in relevant publications
b Populations on the limit of species range
Age classes: j - juvenile, im - immature, v - mature vegetative, g - generative.
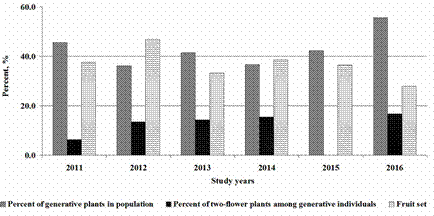
Figure 4 Parameters of reproductive ability of MR population of Cypripedium calceolus in the Republic of Mordovia (Central Russia).
Data on the reproductive ability of MR population showed the relatively low number of two-flower plants in comparison to some populations in other parts of species’ range (Kull 1995, Stetsuk 2013). Fruit-set has been estimated by various authors, it varies considerably throughout species’ range. Levels of fruit set were 33-57% in Belorussia (Stavrovskaya 1984), 4-14% in the Moscow district (Varlygina & Matsenko 1986), 0-25% (7.7% mean over four locations and four years) in Sweden (Nilsson 1979), 11% in Estonia (Kull 1998), 6.8% in Buzuluksky Bor National Park (Stetsuk 2013). Korczyński & KrasickaKorczyńska (2014) found that “in 2013, out of 24 blooming stems 8 fruits were set” in the population near the Lake Kwiecko, without indicating of flowers number at blooming stems. However, the fruit set shown in MR population (36.7% as a mean over study period) was higher than, for instance, in Estonia (Kull 1998), Sweden (Nilsson 1979), the Moscow region of Russia (Varlygina & Matsenko 1986), Buzuluksky Bor National Park (Stetsuk 2013) and Lake Kwiecko in West Pomerania (Korczyński & Krasicka-Korczyńska 2014). The values of fruit set are comparable only with data from Belorussia (Stavrovskaya 1984). As this species is considered as pollinator-limited in some locations (Brzosko et al. 2017, Kull 1998, Nilsson 1979), higher values of the fruit set in comparison with other available data could be explained as a result of presence of habitats suitable for pollinators (wild bees Andrena) in this location. It is in contrast to other studied locations in Mordovia (Fig. 3, Table 3), where percentage of immature and juvenile individuals was extremely low or these were absent.
Conclusions
Our results demonstrate that C. calceolus in the Republic of Mordovia (Central Russia) shows wide ecological variability. Its presence is confined in diverse habitat types: broad-leaved forests, coniferous forests and mixed forests. We suggest the definition of at least one more habitat (pure grassland (see García et al. 2010)) within the range of this species. Based on the comparison of stage spectra of C. calceolus populations both in Mordovia and in other locations within its native range, MR population could be considered as most sustainable and stable in the region due to annually observed juvenile plants, with high percent of mature vegetative and generative individuals in population stage spectrum. Almost annually observed juveniles could be a result of relatively high fruit set found for the population in compared with other available data throughout the whole distribution range of C. calceolus. However, the overgrowth of woody species during natural succession may have a negative impact on the population (Czerepko et al. 2014, Kull 2003, Nicolè et al. 2005). Thus, we can declare on the higher significance of habitat conservation than individual conservation for protection of C. calceolus.
In order to better understand the environmental preferences of C. calceolus, we suggest continue dmonitoring and assessment of its population in Central Russia. The metadata allows us to assume that generalization of jointly obtained demographic, ecological, phytocoenological data will be appropriate for successful conservation and management of C. calceolus.












 uBio
uBio 

