Introduction
Zoophilous flowering plants communicate with pollinators, often specifically so by using specific floral rewards and signalling apparatus, to help ensure pollen transfer between conspecific plants (Wester & Lunau 2016). Although pin-pointing which species are effective pollinators is essential to better understand the functioning and resilience of plant-pollinator networks (Kühsel & Blüthgen 2015, Macgregor, Pocock, Fox & Evans 2015, Weiner, Werner, Linsenmair & Blüthgen 2014), direct observation (Peter et al. 2009, Raguso & Willis 2005, Robertson & Wyatt 1990) is time consuming - even via continuous video monitoring - since the proportion of visited flowers is often low. Moreover, findings obtained via direct observation are typically not fully trustworthy (Suetsugu & Fukushima 2014 a, b). Thus, in general, foraging pollinators are identified via indirect methods, for example the identification of an orchid’s pollinarium on moths’ hairy bodies (Darwin 1877, Maad & Nilsson 2004, Nilsson 1983). Such research is also essential to better understand the potential for hybridization in (orchid) plant systems (Cozzolino & Widmer 2005, Schiestl & Schlüter 2009). Insects visiting orchid flowers can be divided into three main groups: (i) 'flower-visitors, which land on a flower, without any pollinaria attached to their body; (ii) 'potential pollinators’, if pollinia adhere somewhere on the insect’s body (e.g. antennae, head, abdomen), or if they are just likely to carry pollinaria to another flower, and (iii) 'effective pollinators’, when pollinia attached to the insect’s body are eventually deposited on the stigma of another flower (Bournérias et al. 2005, Ruiz 2009).
Moths, an insect group capable of pollinating a wide range of plant species, constitute the majority of nocturnal pollinators (Macgregor et al. 2014). However, as studies on networks of plants and their nocturnal pollen vectors are rare, the role of moths as pollinators is most likely underestimated (Hahn & Brühl 2016). More specifically, moths are known to be the primary pollinators of orchids from the Orchidoideae subfamily (Catling & Catling 1991, Hahn & Brühl 2016). This is also the case for certain orchids of the Platanthera genus, such as the threatened P. praeclara native to the North American prairie, which are highly specialized for pollination by moths (i.e. moth pollination syndrome, or phalaenophily) (Argue 2012, Westwood & Borkowsky 2004).
The Platanthera genus contains ca. 150 species (Karasawa 2003) and is as such the largest genus of northern temperate terrestrial orchids (Hapeman, 1997, Hapeman & Inoue 1997, Wood, Beaman & Beaman 1993). Species belonging to this genus can be found in a wide range of environments, from grasslands to forest understories. They may show ecotypic variation due to co-evolutionary relationships with local pollinators, which are mostly represented by nocturnal moth species (Hapeman & Inoue 1997). Noctuid and sphingid moths represent the majority of Platanthera pollinator species, but there are also some species that are pollinated by beetles, bumblebees, butterflies, flies and even mosquitos (Hapeman & Inoue 1997, Inoue 1985, Nilsson 1983).
Within the framework of our research devoted to Platanthera in Belgium (Esposito, Jacquemyn, Waud & Tyteca 2016), we here focus on the observation of moth visitors of two Platanthera species, namely P. bifolia (L.) Rich. and P. chlorantha (Custer) Rchb. The P. bifolia group is of particular significance because it generated one of the classic textbook examples of presumed selection-mediated co-evolution between orchids and their pollinating insects (e.g., Bateman, James & Rudall 2012, Hapeman & Inoue 1997, Maad & Nilsson 2004, Nilsson 1983, 1985). The flowers of Platanthera are strongly scented, and the scent emission, which happens in the late evening to night, matches the feeding times of many nocturnal moths (Nilsson 1983, Tollsten & Bergström 1993). The two studied species differ in their floral scent composition, which may represent a cue, and which may hence explain the occurrence of different moth visitors (Nilsson 1983, Tollsten & Bergström 1993). Also, the column morphology differs between both species, and a significant difference is also represented by the spur length (Darwin, 1862, Nilsson 1978, 1983, 1985), which plays an important role in pollination effectiveness (Bateman & Sexton 2008; Claessens & Kleynen 2006). Additionally, two morphological traits are especially discriminant between both species: the length of the caudicles and the distance between the viscidia (Nilsson 1983). The latter is particularly significant because it leads to the placement of pollinaria on different parts of the moths’ heads (Claessens & Kleynen 2006, Esposito, Vereecken, Rinaldi, Laurent & Tyteca unpublished, Maad & Nilsson 2004, Nilsson 1983, 1985, Schiestl & Schlüter 2009). Generally, moths will probe deep into the spur to reach the nectar until the head comes into contact with the sticky discs at the base of the pollinaria. In Platanthera, only the visitors that present a suitable scale- or hairless part of the head may be able to touch the viscid disc when the head is forced against the spur mouth (Nilsson 1983). P. chlorantha’s pollinaria generally stick to the eyes of pollinators, and those of P. bifolia to their proboscises (Nilsson 1978, 1983). Consequently, putative hybrids -possessing a column that is morphologically intermediate between the column structures of both species- are expected to interact imperfectly with the flower visitors due to the narrowly delimited surfaces on moths’ heads that are suitable for attachment of viscidia (Claessens & Kleynen 2006, Nilsson 1978). The fact that such intermediately placed pollinaria are not generally found on moths indicates that they regularly become detached, generally from the hairy labial palps of the moths (Nilsson 1983, 1985). Consequently, introgressive hybridization is considered rare and only of temporal and local occurrence. Nevertheless, hybridization has been reported in a few populations, such as in Scandinavia (Nilsson 1985), in southern England (Bateman 2005; Bateman & Sexton 2008), in South Limburg, The Netherlands (Claessens, Gravendeel & Kleynen 2008, Claessens & Kleynen 2006) and in the upper valley of Lavant, Austria (Perko 1997, 2004). In the latter two situations, a large number of intermediate individuals were observed, even in the (quasi-) absence of parent species.
However, in a study that we conducted on two mixed populations of P. bifolia and P. chlorantha in Belgium, molecular data showed that hybridization and genetic admixture occurred only at a very low rate, despite the fact that species of both noctuids and sphingids have been observed visiting both P. bifolia and P. chlorantha (Claessens et al. 2008, Claessens & Kleynen 2006, Nilsson 1983). Moreover, most morphologically intermediate individuals turned out to be genetically identical to P. bifolia, and could therefore not be identified as hybrids (Esposito et al. unpublished).
Here, as a follow-up to this finding, we wanted to uncover the identity of effective pollinators able to visit these intermediate morphotypes in such sympatric populations. As such, we identified flowervisitors of the congeneric orchids Platanthera bifolia and P. chlorantha as well as the morphologically intermediate orchid individuals in these two mixed populations.
Material and methods
Study species. - Both P. bifolia and P. chlorantha species are rewarding, and their nectar is hidden deeply in their long spurs, and hence available only to pollinator species with long proboscises. Their flowering period in central Europe occurs between May and July and is partly overlapping in areas of sympatry (Delforge 2005). Both species show a significant different morphology of the column. P. bifolia displays a small column and two anther pockets that are set almost parallel to each other. Pollinaria are generally transferred by 'massulae’ units (Johnson & Edwards 2000). P. bifolia shows a distance between the viscidia of 0.2 to 1.1 mm and the pollinium shows a very short caudicle (0.2-0.5 mm); these characteristics imply that pollinia will be attached to the proboscis of pollinators. Pollinators of P. bifolia are mostly sphingids (Boberg, Alexandersson, Jonsson, Maad, Ågren & Nilsson 2013, Nilsson 1983, 1988). The column of P. chlorantha is wider with the anther pockets set strongly divergent at the base. Its pollinarium has a relatively long caudicle (1.2-2.2 mm) with a distance between the viscidia varying between 2.3 and 4.9 mm. This particular characteristic is considered an adaptation for attachment to the eyes of the pollinators (Maad & Nilsson 2004), which are mostly represented by noctuid moths (Claessens & Kleynen 2011, Nilsson 1978, 1983, 1985, Sexton 2014, Steen 2012). The distance between the viscidia of intermediate individuals is, on average, larger than in P. bifolia and smaller than in P. chlorantha (1.3-2.3 mm).
The visual assessment method that allows the assignment of the visited Platanthera species based on the morphology of pollinaria and on their position on pollinators’ head was proposed firstly by Nilsson (1983) and confirmed by the study of Claessens et al. (2008).
Study area and sampling. - The study was performed in the Calestienne region in southern Belgium, in 2013 and 2014. Floral visitors were recorded during the peak flowering times in two sympatric Platanthera populations on the 22nd of June 2013, again in one of these on the 27th of June 2013 and finally in the other sympatric site on the 3rd of June and the 4th of July 2014. Light traps were running from 21:00 h and checked early next morning from 04:00 h onwards. One of the mixed populations was located on a calcareous grassland (Tienne de Botton), while the other was located in a light birch-ash wood (Bois Niau). In order to catch pollen-vectors, we utilised light traps that have proven to be highly suitable for sampling moth communities (Heath 1965, Merckx et al. 2009a, b, Merckx, Marini, Feber & Macdonald 2012a, Merckx et al. 2012b, Young 1997). Sampling moths with light traps has also some additional advantages in terms of cost and ease of use, compared to video monitoring for instance (Steen & Mundal 2013).
Two light traps were deployed for each of the sympatric zones, where two Platanthera species and the morphologically intermediate individuals were growing intermixed, hence possibly sharing pollinators. Light traps were placed in close vicinity (3-10 m) to inflorescences in good flowering conditions. Moths were sampled using Heath pattern actinic light traps (6 W), which operate on the 'lobster-pot principle’, whereby individuals are drawn to an actinic tube, which is secured vertically between baffles, fall unharmed down a funnel, and rest inside the trap (Fig. 1). At dawn, captured moths were checked for the presence of Platanthera pollinaria by visual assessment. To facilitate the identification of the species/type of pollinaria we utilised a Peak scale magnifier. Moreover, during two observations nights, we photographed moth individuals, which were seen visiting Platanthera species without the support of light traps. Moths bearing pollinaria were identified, whilst the type and number of pollinaria was accurately checked. Specifically, we measured the length of the caudicle of the pollinaria attached to the moths’ heads. Additionally, moths carrying pollinaria were photographed with a digital camera (Canon Eos 7D, Nikon D-200). We lumped data obtained with and without light trapping for analyses.
Results
Table SI1 gives the total number of nocturnal moths light-trapped during the observation nights at the sympatric sites of Botton and Bois Niau. On the whole, seven individuals from two mediumsized crepuscular-nocturnal species were carrying Platanthera pollinaria (Table 1).
Generally speaking, these observations show that pollinaria were carried according to expectations, i.e., one P. chlorantha pollinarium on the eye, two P. bifolia pollinaria on the proboscis, and one or two intermediate’s pollinaria on the cheeks. The measurement of the length of each pollinarium determined the assignment (according to Nilsson’s criteria 1983) to Platanthera species previously visited, and also confirmed the species attribution according to the position of pollinaria on the moths’ heads (measurements of pollinia’s length not shown). These results also show that, in both sympatric locations, although the majority of species caught were belonging to the Geometridae family (Table 2), we only observed individuals of the Noctuidae family carrying Platanthera pollinia. More specifically, the total amount of moth species belonging to the Noctuidae family found with the pollinaria attached on the body was two species out of six. The only noctuid species found to carry intermediate’s pollinaria turned out to be Cucullia umbratica.
Besides these light trap experiments, during two observation nights on 3rd June and 4th July 2014 at the Botton site, we managed to take a picture of a Cucullia umbratica moth visiting a P. chlorantha inflorescence with one pollinium attached to the cheek and (probably) two to the proboscis (Fig. 2F). Another visitor was also observed; this was Noctua pronuba with a pollinarium of P. chlorantha on the eye (Fig. 2G).
Table 1 Overview of moth individuals observed carrying orchid pollinaria during four nights of monitoring. Superscript numbers in front of the moth species name indicate different individuals of moths.
| Date | Site | Plant species | Number pollinaria | Moth species | Position pollinaria | Figure |
|---|---|---|---|---|---|---|
| 22/06/13 | Botton | P. chlorantha | 1 | 1 Cucullia umbratica | Eyes | 2-D |
| - | - | Intermediate | 2 | 2 Cucullia umbratica | Cheeks | 2-E |
| - | - | P. bifolia | 2 | 3 Cucullia umbratica | Proboscis | 2-C |
| 22/06/13 | Bois Niau | P. bifolia | 2 | 1 Autographa gamma | Proboscis | - |
| - | - | P. chlorantha | 1 | 2 Autographa gamma | Eyes | - |
| 27/06/13 | Bois Niau | P. bifolia | 2 | 1 Cucullia umbratica | Proboscis | 2-A |
| - | - | P. bifolia | 2 | 1 Cucullia umbratica | Proboscis | 2-A |
| - | - | P. bifolia | 2 | 2 Cucullia umbratica | Proboscis | 2-B |
| 3/6/14 | Botton | Intermediate | 1 | Cucullia umbratica | Cheeks | 2-F |
| - | - | P. bifolia | 2 | Cucullia umbratica | Proboscis | 2-F |
| 4/7/14 | Botton | P. chlorantha | 1 | Noctua pronuba | Eyes | 2-G |
Discussion
The position and shape of the pollinaria attached to the caught moths proved sufficient to identify which orchid species had been visited, even if the visits themselves were not observed. One of the three observed moth species, Cucullia umbratica, here observed with pollinaria of P. bifolia and P. chlorantha, was already known to be a visitor of both Platanthera species (e.g. Nilsson 1978, 1983). However, contrary to the assumption of Nilsson (1983), who states that efficient transport of pollinaria on cheeks is impossible for putative hybrids, our observations in one of the sympatric populations show that the species visits flowers of intermediates, because their pollinaria were firmly stuck to the cheeks. This result corroborates earlier observations by Claessens et al. (2008) who captured the images of C. umbratica in the act of removing pollinaria via their proboscises from putative hybrids in the Netherlands. We succeeded in photographing one individual of C. umbratica approaching an inflorescence of P. chlorantha with three pollinaria attached to its cheeks (i.e. from intermediate orchid individuals; see Fig. 2F). This observation may represent a proof of the potentiality of intermediate morphotypes to act not only as pollen recipients but also as pollen donors. In this case, crossing between intermediate forms could be effective (Claessens et al. 2008). It thus appears there is scope for hybridization and subsequent introgression, but this needs further testing. Furthermore, during our observations in one of the mixed populations (Bois Niau), we recorded two Autographa gamma individuals carrying both P. bifolia and P. chlorantha pollinaria on the proboscis and on the eye, respectively. The common and widespread noctuid A. gamma had already been described as one of the prominent pollinators of P. chlorantha in Sweden (Nilsson 1978), in south-central Scotland (Sexton & McQueen 2005) and in Spain (Ruiz 2009). Moreover, this moth species had also already been observed visiting P. bifolia (Plepys, Ibarra, Francke & Lofstedt 2002) and carrying its pollinaria (Ruiz 2009). Noctua pronuba too, which in this study was photographed with one P. chlorantha pollinium on the eye (Fig. 2G), had already been observed visiting P. chlorantha in Sweden (Hammarstedt 1980), in Central Scotland (Sexton 2014) and in the Iberian Peninsula (Ruiz 2009).
Thus, contrary to the literature (Nilsson 1983), which mentions mostly sphingids (especially Deilephila species) as the potential vector of hybridization, our observations show that it may well be possible that both orchid species are mainly pollinated by noctuid moths, and more specifically by C. umbratica and A. gamma effectively carrying pollinaria of both Platanthera species, with C. umbratica even observed carrying pollinaria of morphologically intermediate individuals. However, the dominance of these specific noctuids may be a matter of local and regional occurrence, influenced too by the availability of vegetation types, with other Cucullia and Plusiinae species being pollinators at sites in other regions (Hammarstedt 1980, Nilsson 1983, Sexton 2014).
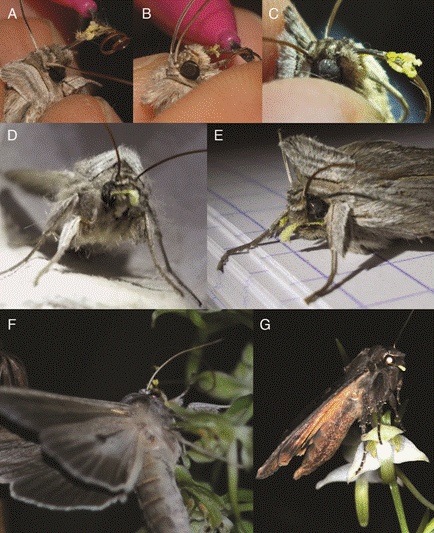
Figure 2 A-B: Bois Niau: two individuals of Cucullia umbratica with two pollinaria of P. bifolia on the proboscis (pictures D. Tyteca); C-D-E: Botton: three individuals of Cucullia umbratica, one with P. bifolia pollinaria on the proboscis, one with one P. chlorantha pollinarium on the eyes, and the other with two intermediates’ pollinaria on the cheeks (pictures F. Esposito); F-G: Botton: Cucullia umbratica visiting P. chlorantha inflorescence with one pollinarium stuck to the cheek (coming from intermediate plant) and two to the proboscis (coming from P. bifolia), and Noctua pronuba with one P. chlorantha pollinarium stuck to the eye (pictures Guy Deflandre).
Table2. Abundance of all macro-moth species, with indication of the family they belong to, light-trapped at the two sympatric sites.
| Site - Date | N. ind. | Moths species | Moths family |
|---|---|---|---|
| Botton 22/6/13, 3/6/14 4/7/14 | 2 | Arctia villica | Erebidae |
| 1 | Eilema griseola | ||
| 2 | Laspeyria flexula | ||
| 1 | Spilosoma lubricipeda | ||
| 1 | Anticollix sparsata | Geometridae | |
| 2 | Campaea margaritaria | ||
| 1 | Camptogramma bilineata | ||
| 1 | Chiasmia clathrata | ||
| 1 | Hylaea fasciaria | ||
| 4 | Hypomecis punctinalis | ||
| 1 | Lomographa temerata | ||
| 1 | Macaria liturata | ||
| 1 | Opisthograptis luteolata | ||
| 1 | Thera obeliscata | ||
| 2 | Thera spec. | ||
| 1 | Xanthorhoe designata | ||
| 1 | Xanthorhoe montanata | ||
| 2 | Pharmacis lupulina | Hepialidae | |
| 1 | Dendrolimus pin | Lasiocampidae | |
| 1 | Agrotis exclamationis | Noctuidae | |
| 3 | Apamea sublustris | ||
| 2 | Autographa gamma | ||
| 4 | Cucullia umbratica | ||
| 1 | Noctua pronuba | ||
| 1 | Pachetra sagittigera | ||
| 2 | Rusina ferruginea | ||
| 1 | Ptilodon capucina | Notodontidae | |
| 1 | Laaothoe populi | Sphingidae | |
| Bois Niau 22/6/13, 27/6/13 | 1 | Habrosyne pyritoides | Drepanidae |
| 1 | Thyatira batis | ||
| 1 | Herminia grisealis | Erebidae | |
| 2 | Spilosoma lubricipeda | ||
| 3 | Spilosoma lutea | ||
| 3 | Angerona prunaria | Geometridae | |
| 2 | Cabera exanthemata | ||
| 2 | Campaea margaritaria | ||
| 1 | Cepphis advenaria | ||
| 2 | Colostygia pectinataria | ||
| 2 | Dysstroma truncata | ||
| 1 | Ecliptopera capitata | ||
| 1 | Ecliptopera silaceata | ||
| 1 | Eupithecia haworthiata | ||
| 2 | Hypomecis roboraria | ||
| 2 | Ligdia adustata | ||
| 1 | Lomaspilis marginata | ||
| 2 | Macaria liturata | ||
| 1 | Melanthia procellata, | ||
| 1 | Parectropis similaria | ||
| 3 | Xanthorhoe montanata | ||
| 3 | Pharmacis lupulina | Hepialidae | |
| 2 | Calliteara pudibunda | Lymantriidae | |
| 1 | Abrostola tripartita | Noctuidae | |
| 1 | Apamea sublustris | ||
| 1 | Agrotis exclamationis | ||
| 2 | Autographa gamma | ||
| 1 | Autographa pulchrina | ||
| 2 | Cucullia umbratica | ||
| 1 | Deltote pygarga | ||
| 1 | Euplexia lucipara | ||
| 1 | Hoplodrina octogenaria | ||
| 1 | Hypena proboscidalis | ||
| 1 | Lacanobia thalassina | ||
| 1 | Ochropleura plecta | ||
| 1 | Drymonia obliterata | Notodontidae | |
| 1 | Gluphisia crenata | ||
| 2 | Phalera bucephala | ||
| 1 | Stauropus fagi |
Most noctuids and sphingids are described as strong fliers, able to cover large distances (Nieminen & Hanski 1998). Specifically, A. gamma and N. pronuba have been recorded to move distances of several hundred kilometres (Chapman et al. 2010, 2012, Hu, Lim, Reynolds, Reynolds & Chapman 2016, Waring, Townsend, & Lewington 2009). Their high mobility, both in terms of routine, daily movements (Slade et al. 2013) as in terms of (partial) migratory behaviour, possibly may have important effects on pollen dispersal ability and the extent of hybridization (Brys, Broeck, Mergeay & Jacquemyn 2014). Reproductive isolation as well as the level of introgression are generally controlled through three kinds of integrated interactions: temporal, ethological (i.e. pollinators reacting to floral fragrances and to nectar availability) and morphological (i.e. interactions between the morphology of pollinators and the morphology of columns and spurs) (Esposito et al. unpublished, Nilsson 1983, 1985). Within the framework of the Platanthera study system, we hypothesize that the mechanical barrier preventing (or reducing) effective hybridization involves that the pollinia of P. chlorantha may often not be adequately placed in order to fit the position of P. bifolia’s stigma. This hypothesis contrasts with the alternative hypothesis, which suggests that the mechanical barrier may be due to the improper placement of pollinia from intermediate plants on visiting moths (Nilsson 1983). However, both hypotheses may fit with the rarity of real hybrid individuals, but does not explain why those intermediate plants, which appear to belong mainly to the gene pool of P. bifolia, appear in mixed populations. A previous hypothesis was formulated to give an explanation for the presence of these intermediate individuals (Esposito et al. unpublished). It seems that among those intermediate plants, the individuals tending towards P. chlorantha (which have a greater distance between their viscidia, and which flower earlier) are positively selected in order to attract and exploit P. chlorantha pollinators too. We have evaluated the validity of this hypothesis by exploring the effect of morphological traits on phenotypic selection through observing the relationship between plant trait expression and male versus female fitness, as a result of the interactions with pollinators (Esposito et al. unpublished). Thus, it seems that there is higher morphological variability within P. bifolia when the species comes in sympatry with P. chlorantha, probably because there is a higher selective pressure exerted by P. chlorantha’s pollinators. However, we do not know if Platanthera species just respond plastically to environmental conditions or whether they are in a process of early speciation and specialization in response to local pollinators.
In conclusion, although our study shows that noctuids have the potential to cause hybridization in Platanthera, more observational and genetic research is needed. Studies that test successful pollen deposition across species, whilst assessing pollen dispersal distances, are essential in order to eventually (dis)prove hybridization is actually happening, and to hence better understand Platanthera-pollinator networks.












 uBio
uBio