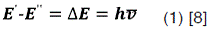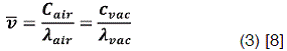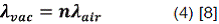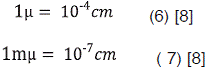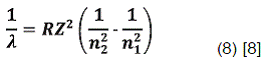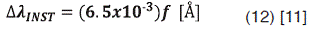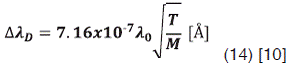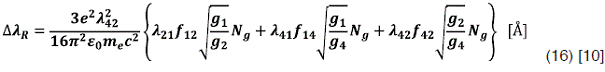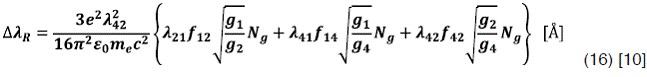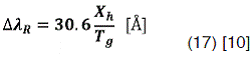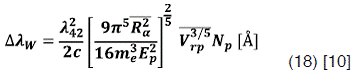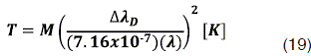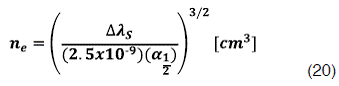Introduction
Recently atmospheric pressure plasmas have become a topic of great interest for a wide range of applications in different branches of industry. In these plasmas the electron temperature is far higher than the temperature of the heavy particles [1]; elastic collisions of the electrons are not effective in contrast to collisions of heavy particles however it can transfer energy to other processes such as ionization, activation or dissociation of molecules this explains the great interest in this type of plasmas.
Over the past 20 years, there has been growing interest in developing this type of devices, particularly for biomedical applications such as bacterial inactivation [2, 3], wound healing [4], dental bleaching [5]; atmospheric plasmas are also of great interest in the industry, improve adhesion for inks, paints, and coatings are examples of plasma applications in order to improve surface properties, changes on the surface energy of any material is one of the purposes of applying plasma [6].
Recently an atmospheric pressure plasma-JET and a parallel plate plasma reactor for biomedical and industrial applications has been developed in our Laboratory1, so there is a need to venture into the application of passive techniques to measure temperature and density in the plasma, the use of passive spectroscopy does not perturb the internal kinetics of the discharge since the observation are being carried out [7].
Basics on Spectroscopy
Atomic Spectra
Atomic spectra are principally concerned whit the interchange of energy between the atom and electromagnetic radiation, where the exchange may be associate on the simplest model whit a valence electron changing its orbit. Energy could be added to the radiation field (emission spectra) or also absorbed from it (absorption spectra).
The actual change in energy (ΔE) between the energy levels in an atom is related to the frequency of the radiation absorbed or emitted by the equation:
Where h is Planck’s constant and is the frequency in cycles per second, E' is the total energy of the atom in its higher state and E" in the lowest state. For cases under study is of particular interest hydrogen atom because of its presence in plasma discharges at atmospheric pressure; there are many possible energy levels taking into account the values of energy levels corresponding with the principal quantum numbers ( n ) 1, 2, 3, 4, 5, there are E1, E2, E3, E4 and E5 respectively.
If hydrogen is present in an electric discharge some of the molecules split up into atoms and some of these hydrogen atoms may reach an excited electronic state that could have n values greater than one. Some of the exited atoms lose the whole of theirs excess energy, returning to the lowest level (n=1, the ground state) and will emit radiation whit frequencies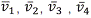 some electrons return to the n=2 state and produce another set of frequencies
some electrons return to the n=2 state and produce another set of frequencies 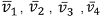 these different frequencies can be sorted out either by passing the emission beam through a prism or by a diffraction grating, the separation of the radiation into its component frequencies gives the spectrum of the element.
these different frequencies can be sorted out either by passing the emission beam through a prism or by a diffraction grating, the separation of the radiation into its component frequencies gives the spectrum of the element.
Spectroscopy is a non-intrusive method used to analyze electromagnetic radiation emitted or absorbed from a plasma source in order to obtain information about the plasma such as: electron density, state densities of the excited species, collisional electron-atom, atom-atom and ion-atom effects, energetic distribution of the species, temperature of the species, charge transfer between the components of the plasma, rotational structure of molecules and even electric and magnetic fields properties.
For these studies is common to consider the term wavelength instead frequency, these two terms are related by the equation:
The values of λ and c depend slightly on whether the measurements are made: in vacuum or in air; the frequency, however, is in each case given by:
If measurements are made in air for the wavelength may be corrected to 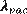 by:
by:
Where n is the refractive index of air at that particular wavelength
Electromagnetic Spectrum
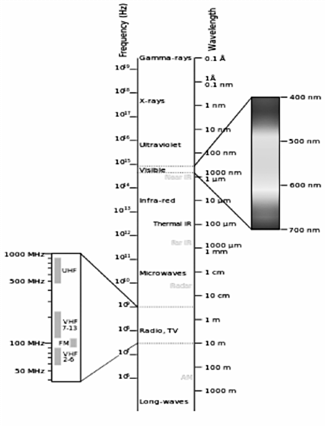
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The electromagnetic spectrum as see in Fig 1. extends from below the low frequencies used for modern radio-communication to gamma radiation at the short-wavelength (highfrequency).
The units employed to describe wavelength depend on the spectral regions concerned. Ångström (Å) units are normally used for atomic spectra, where 1 Å = 10-8 cm (5), other systems of units employed for recording wavelength are the micron (µ) and the millimicron (mµ)
The study of plasma emission is focusing in the visible part of the spectrum although other spectroscopic techniques can be located in the region of ultra-violet (UV) or infra-red (IR) zones.
Hydrogen Spectrum
One of the best approximation to hydrogen spectral series can be explained in terms of principal quantum number values. However, when the hydrogen lines are examined with a spectrograph of great resolving power, splitting of the lines may be detected. The emission spectrum of atomic hydrogen is divided into a number of spectral series, where wavelengths are given by the Rydberg formula:
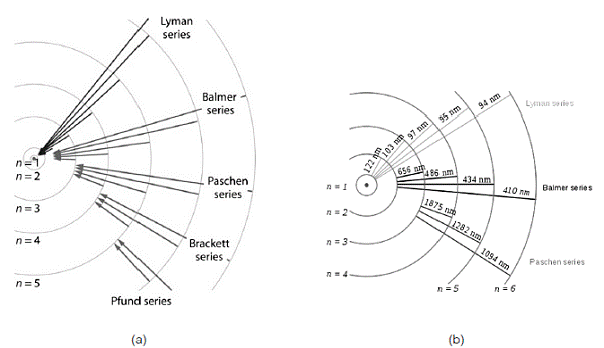
R is the Rydberg constant (approximately 109 677.59 cm-1),λvac is the wavelength of the light emitted in vacuum, Z is the atomic number, and n1 and n2 are integers representing the energy levels involved such that n1< n2. Is important to emphasize that all observed spectral lines are due to electrons moving between energy levels in the atom. Fig. 2 shows the emission transitions for some series of hydrogen atom.
Some of these series of emissions are important for the calculation of some plasma parameters, especially at atmospheric pressure, for example Balmer Series which expected values in Å are given by Table 1.
Table 1 Balmer Series for hydrogen atom [8]
| Line | [67]Wavelength [Å] |
| Hα | [70]6562.79 |
| Hβ | [73]4861.33 |
| Hγ | [76]4340.47 |
| Hδ | [79]4101.74 |
| Hε | [82]3970.07 |
| Hζ | [85]3889.05 |
| Hη | [88]3835.39 |
| Hθ | [91]3797.90 |
| Hι | [94]377.63 |
Line broadening mechanisms
Even if the emission lines are caused by quantized level transitions, they possess a defined width and shape that are associated with mathematical functions, the line shape of profile of a spectral line is dependent of the mechanism resulting in a total broadening that may be related to the collisional processes occurring in plasma or independent of it for example in the caused by the spectrometer or the nature of transition[10]. Table 1. shows broadening associated due particle collision and Lorentzian profile while Table 2. shows other types of broadening due different causes and also associated to Gaussian profile.
Table 2 Broadening due particle collisions [10]
| Broadening Type | [104]Collisions between |
| Resonance | [107]Identical particles |
| Van der Walls | [110]Different neutral particles |
| Stark | [113]Charged Particles |
Table 3 Broadening due other causes[10]
| Broadening Type | [118]Due to: |
| Natural | [121]Results from Heisenberg incertitude principle |
| Instrumental | [124]Results from measuring spectrometer which has a finite resolution |
| Doppler | [127]Difference in speed of particles |
Corresponding profiles are superimposed by convolution when more than one broadening effect is at work so the line-shape or profile of a spectral line is dependent of the various mechanisms that causes the total broadening. That’s is why is correct to say that spectral lines suffer a number of different broadening mechanisms resulting in a total broadening [10].
Because the sum of several different mechanisms associated with specific mathematical functions (Gaussian and Lorentzian profiles) is necessary the use of a line profile suitable for the resulting measurements. The Voigt profile it’s a common line profile used in spectroscopy and is a convolution of Gaussian and Lorentz profiles, whose function is given by:
Where wL and wG are the FWHM (Full width at half maximum) of the Lorentzian and Gaussian component respectively. This components are specifically given by:
A small description for mentioned broadening mechanism in Tables 2 and Table 3 is given below, referring the FWHM for each of them:
Instrumental Broadening
The instrumental width is given by:
Where f is the width of the slits in µm. This is caused by limits of the measuring spectrometer which has a finite resolution. It is important to check with the provider of the instrument the value of 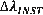 as this may vary from instrument to instrument
as this may vary from instrument to instrument
Natural Broadening
The natural broadening is caused by the finite lifetime of exited states and also can be determined by Heisenberg’s uncertainty relation. [11] is given by:
Since its value is in the order of ~10-3 it can be neglected in some cases.
Doppler Broadening
This broadening is caused due the thermal velocity of the emitting atoms. I’ts possible to assume that the velocity follows a Maxwellian distribution and it depends only on their kinetic temperature T. So 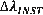 is given by:
is given by:
Where M is the mass of the radiating atom in atomic mass units, λ0 is the central wavelength in nm and T the temperature of the radiating atoms which in some cases may be equal to the gas temperature.
Stark Broadening
This broadening is caused by Coulomb interactions between the radiator and charged particles present in plasma, also ions and electrons are responsible of this broadening but electrons presents the major effect due to their higher relative velocities. So 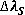 is given by:
is given by:
Where 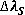 = 0.0783 [Å/cgs][6]
= 0.0783 [Å/cgs][6]
Resonance Broadening
This broadening is caused by the interaction of the emitting atoms with atoms in ground state.
Usually, in hydrogen plasmas the three perturbing transitions considered are 1->2, 1->4 and
Table 4 Constants for 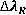 calculations [10]
calculations [10]
Also 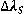 can be related whit the hydrogen atoms mole fraction Xh and the gas temperature Tg by the following expression:
can be related whit the hydrogen atoms mole fraction Xh and the gas temperature Tg by the following expression:
Van der Waals Broadening
This broadening is caused by perturbers that do not share a resonant transitions with the radiating atom. 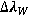 is given by:
is given by:
Where 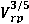 is the relative speed of the radiating atom and the perturber, Ep is the energy of the first exited state of the perturber connected whit its ground state by an allowed transition, and
is the relative speed of the radiating atom and the perturber, Ep is the energy of the first exited state of the perturber connected whit its ground state by an allowed transition, and 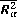 is a matrix element.
is a matrix element.
Spectroscopic study of atmospheric plasma
Spectrometer
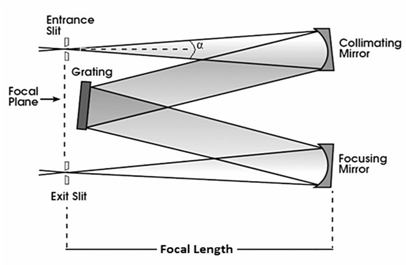
The instrument that records the relative intensities of the wavelengths that are present in a light beam, over a certain wavelength interval is known as spectrometer, its basic structure can be observed in Fig.3
The spectrometer also has a dispersing element that spatially separates the various wavelength components entering through the Entrance Slit; in most practical situations this is a reflection grating, which has a mirror-like surface with a periodic structure and at the end in the Exit Slit it has a detector to collect the dispersed light out of the device. The output signal is then analyzed to determine the relevant parameters of plasma
Some of the most important parameters of a spectrometer are: Focal length, difraction grating, resolution, dispersion and aperture. There are multiple tutorials and guides that explain the physics of these devices and help to find a number of key parameters for the calculation for future calculations, some of these parameters are inherent in constructive features of the device used, so the reader is invited to search for relevant literature.
Plasma classification
Plasma, a quasi-neutral gas, is considered to be the fourth state of matter, following the more familiar states of solid, liquid & gas and constitutes more than 99% matter of the universe. It is more or less an electrified gas with a chemically reactive media that consists of a large number of different species such as electrons, positive and negative ions, free radicals, gas atoms and molecules in the ground or any higher state of any form of excited species [12].
In order to obtain information about the plasma is important to consider some characteristic of de discharge, plasmas can exist over an extremely wide range of temperature and pressure.
One of the most useful classifications for plasmas is depending on their temperature; plasmas can be distinguished into two main groups i.e., the high temperature or fusion plasmas and the low temperatures or gas discharges.
A typical classification and parameters of different kinds of plasmas is given in Table 5.
| [205] [206] | |||||
|---|---|---|---|---|---|
| Plasma | [207]State | [208]Notes | [209]Example | [210] [211]||
| High temperatura plasma [212] (Equilibrium plasma) | [213]Te≈Ti≈Tg [214] Tp=106-108K ne≥1020m-3 | [215][216] | [217]Laser Fusion [218] Plasma [219] Tokamak Plasma | [220] [221]||
| Low temperature plasma | [222] [223]|||||
| Plasma | [224]State [225] | [226]Notes [227] | [228]Example | [229] [230]||
| Thermal plasma(Quasi-equilibrium plasma) | [231](Te≈Ti≈Tg)≤ 2x104K ne≥1020m-3 | [232][233] | Arc plasma, plasma torches, RFinductively coupled discharges | [234] [235]||
| Non thermal plasma(Non-equilibrium plasma) | [236][Te>>(Ti≈Tg)]=300…103Kne≈1010m-3 | [237]Tg≈TrTv>T r (indicates the non-equilibrium in plasma) | [238][239] | Glow, corona, APPJ, DBD, MHCD,OAUGDP, plasma needle etc | |
Where:
Te=Electronic Temperature
Ti=Ionic Temperature Tg=Gas Temperature ne=electronic density
Tv=Vibrational Temperature Tr=Rotational Temperature
Spectroscopy study of atmospheric plasma
The shape and width of a spectral line emitted by the plasma are a consequence of the processes that happen in the discharge. Moreover, the optical device used in the laboratory to register the radiation introduces additional broadening on its profile [7].
The spectral line profile can be approached to a Voigt function (Voigt profile) at atmospheric pressure conditions. This function is the result of the deconvolution of a Gaussian function with a Lorentzian function, their broadenings being ∆λG and ∆λL, respectively. The Gaussian part of the profile is due to the Doppler (∆λD) and the instrumental broadenings (∆λI); the Lorentzian part to the Stark (∆λS) and the van der Waals (∆λW) ones. To unfold the Voigt profile into its Lorentzian and Gaussian components it should be used adequate software [7].
It’s also important to consider other broadenings that contribute to the total profile width in order to determine if it can be negligible or not. Below is a quick explanation about the calculation of parameters of plasma using the spectra generated.
Gas temperature
This temperature (Tg) corresponds to the measurement of energy acquired by heavy particles (atoms and ions) of discharge principally by collisions with the plasma electrons.
As shown in Table 5. the gas temperature may approach the rotational temperature, especially that emitted by certain molecular species as N2 C2 CN and OH, many of those molecules are present in discharges at atmospheric pressure, there are many software available for free for the simulation and comparison with experimental data, so it is possible to determine the gas temperature by measuring rotational temperature of one of these species and then compare with simulation data.
In some cases the ro-vibrational spectra of the molecular species are too weak. Then, another possibility is to determine Tg directly from the Doppler or the van der Waals broadenings of the spectral lines emitted by the plasma because each one of these broadenings is a function of the gas temperature [7].
When the instrumental broadening dominates the Gaussian component of the spectral line, the Doppler broadening cannot be used to obtain the gas temperature value [7].
Electron Temperature
This (Te) value corresponds to the energy of the plasma electrons and being use in the ionization an excitation processes that take place in the discharge. In order to obtain information about electronic temperature of the plasma there is possible to use the Hβ line, from the Hydrogen Balmer series (transition from n=4 to n=2 levels) the experimental determination of the temperatures can be achieved by fitting experimental spectra with simulated ones assuming a Maxwellian distribution of the atoms[11]given by Ec.9
In order to calculate the temperature is necessary to use the expression of the gaussian width given by Ec. 11 and consider the instrumental broadening given by Ec.12. From the Doppler broadening (Ec. 12) is possible to estimate temperature using the following equation:
Electron density
Since electrons control the processes of excitation and ionization that take place in the discharge directly or through other processes, this value ne is one of the most important plasma parameter to consider.
In order to calculate the electron density of the plasma is necessary to use the Stark broadening equation, inverting equation 15 is possible to get ne:
In plasmas with electron density value higher that 1015 cm-3 the contribution of the van der Waals broadening to the Lorentzian width can be considered negligible [ 7]; So it’s possible to assume that the most relevant contribution for 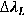 come from the Stark broadening (
come from the Stark broadening ( 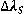 ) also for low pressure an atmospheric discharges., therefore, ne is obtained by inserting the Lorentzian width component obtained from the fitting process in equation.
) also for low pressure an atmospheric discharges., therefore, ne is obtained by inserting the Lorentzian width component obtained from the fitting process in equation.
However, for plasmas with electron density lower than 1015 cm-3 it is necessary to calculate the contribution of the van der Waals broadening to the Lorentzian width and eliminate this broadening from that one [14].
Conclusions
A method and introduction to measure atmospheric plasma parameters (densities and temperatures) through optical spectroscopy have been described, is very important to know beforehand instrumental limitations and plasma characteristics in order to determine whether it is possible to apply the method described.
According to the power source and type of reactor used to generate a plasma discharge, it’s easy to establish a classification according to the possible plasma temperature, them is recommended to apply the classification criteria indicated in Table 5 in order to simplify the analysis to be performed.
For Non thermal plasma (non-equilibrium) the rotational temperature, could be emitted by certain molecular species as N2 C2 CN and OH, there are many database and spectral simulation software tools (like LIFBASE) available for the simulation and comparison with experimental data, so it is possible to determine the gas temperature by measuring rotational temperature of one of these species and then compare with simulation data.