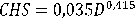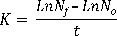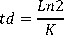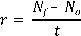Introduction
The larvae of Lutjanus guttatus as well as many marine fish are characterized by their small size, which makes them vulnerable to both rearing conditions and the quality and quantity of live food to be provided at the hatchery (Aristizábal and Suárez, 2006); this is why the first feeding is considered the bottleneck of the fish farming industry (Dhert et al., 2001).The rotifer Brachionus sp. has been listed as one of the most important fish farming food resources (Suantika et al., 2001), by size (Hagiwara et al., 2007), swimming speed and his ability to remain suspended in the water column (Fielder et al., 2000), so it is necessary to establish a massive stable supply enabling mass production of these larvae for consumption in the laboratory (Dhert et al., 2001).
This has encouraged to research on the conditions that optimize the growth of populations of this organism (Kostopoulou et al., 2006; Sayegh et al., 2007; Papakostas et al., 2007; Kobayashi et al., 2008; Larsen et al., 2008; Yin and Zhao, 2008; Cavalin and Weirich, 2009; Mahmoudzadeh et al., 2009; Qi et al., 2009), specifically, may studies related to the addition of pure oxygen as support for rotifer cultures (Yoshimura et al., 1996; Dhert et al., 2001; Yoshimura et al., 2003), however, is necessary to evaluate the economic conditions as well as staff availability and infrastructure before deciding the technique with which to work, as noted by Suantika et al. (2003) in their study on rotifers production progress.
The aim of this study was to optimize the production of rotifers Brachionus plicatilis according to economic and technical conditions developed at the Pacific Marine Park and improve his technology on marine-culture in Costa Rica.
Materials and methods
An own strain of Brachionus plicatilis from the Pacific Marine Park stock was used, which is located in the city of Puntarenas (9,97° N 84,82°W), Costa Rica at an altitude of 0 meters above the sea level. The experiment consisted of three replicates both to evaluate the population growth curve in the system with addition of pure oxygen and the control treatment (no addition of pure oxygen). The first test, ha both oxygen added as 99% and oxygen by an electric aeration blower driven, control culture only had air pushed by the blower. To conduct the experiment transparent fiberglass tanks of 500 liters in volume were used, located under covered roof of transparent polycarbonate sheets with an entry of light during day hours (5000 lux). Cultures were initiated with a density of 200 rotifer . ml-1.
Water quality
To maintain the quality of seawater, ultraviolet rays were irradiated filtered to 1 µm. To collect flocs of organic matter, suspended absorbent fabrics were installed in tanks of 2 x 0.2 m and a skimmer was installed to collect organic remains. The bottom of the tanks were cleared to maintain the cleanliness of the crop and finally 0.75 g . day of probiotic Epicin pond were added to the tanks (Bionetworks Epicor Inc., Eastampton, NJ 08060).
Feeding
The daily maintenance diet was based on dry yeast (Saccharomyces cerevisiae) and addition of micro-algae Nannochloropsis oculata achieving the recommended density in the tank by Dhert et al. (2001) with 200,000 cells . ml-1. Regarding the amount of yeast used, the amount was based on the formula of Suantika et al. (2000), which corresponds to:
where:
CHS = weight of food (g)
D = density of rotifers ml-1
V = volume (l)
The way to deliver yeast to rotifers was based on Benetti et al., (2008), which consists on preparing them in coolers of 20 liters each with constant aeration, decreasing the temperature with ice down to 10°C, adding 1.25 g of probiotic Epicin ponds and finally deliver this to rotifers by continuous drip for 24 hours using a peristaltic pump of Chem Feed ® brand (Blue-White Industries, Huntington Beach, California 92649).
Physicochemical parameters
The oxygen dewar was regulated to keep on the rotifer cultures an oscillating oxygen interval between 5 and 6 mg . l-1. The temperature and dissolved oxygen (DO) was registered three times a day with a YSI oxygen meter model 550A. Cultures were maintained in a salinity range between 20 and 22 ppt. In addition the pH and the concentration of ammonia (NH3 / NH4 +) were recorded daily using a colorimetric test Aquarium Pharmaceuticals (Fishcare North America, Inc. Hamilton, California, PA 18914).
Population parameters
Density (ind . ml-1) and fecundity (eggs . rot-1) of Brachionus plicatilis, was calculated base on ten counts daily (1 ml aliquots) of live rotifers using a stereoscope “Optimum model ZM-160 AT”. Also when the cultures showed their highest population density values calculation of the instantaneous growth rate (day -1), doubling time (day -1) and crop yield (rot . l-1 . day-1) were performed, which are given by the following formulas:
Instantaneous growth rate (Suantika et al., 2003):
Doubling time (Vallejo et al., 1993):
Crop yield (Vallejo et al., 1993):
where:
Nf = final number of ind . ml-1 No = initial number of ind . ml-1 t = time in days.
Length and lorica width (microns)
Five daily rotifers were taken randomly from each tank to measure with the microscope (H-903 model Optima) both the length and lorica width (body wall). Immobilization was obtain by using lugol reagent.
Economic Analysis
Concerning economic analysis, the items of consumption that comprise production costs for 2,000 million rotifers (oxygen treatment and control) were defined, these were: power consumption, oxygen, algae, yeast, probiotic and labor. As a final activity, the percentage of total feed costs for a pond of 4 tons was calculated; based on the methodology of Boza et al., 2008 which used a spawning of 50,000 and getting at day 67 after hatching a survival of 1.5% of Lutjanus guttatus larvae which implies: the use of rotifers, Artemia cysts, algae and Otohime as formulated feed (Reed Mariculture, Inc. Hamilton Ave, Suite 100 Campbell, CA 95008).
Results
Physico-chemical conditions
It was observed that in oxygen system, the DO was maintained at an average range from 6.47+0.37 and 4.62+0.68 mg . l-1 throughout the culture time, while in the control treatment values declined 5.52+0.88 to 0.99+0.52 mg . l-1 at the last day of culture, showing significant differences from the second day (ANOVA P <0.05) between treatments. The pH was stable in both cultives without significant variations (ANOVA P> 0.05) throughout the investigation, with values between 7.46+0.10 and 7.5+0.20 mg . l-1. The NH3/NH4+ showed an increase without significant changes between the two systems (ANOVA P> 0.05), reaching values up to 8+0.1 mg . l-1 on the last day of treatment with a temperature fluctuation between 29.50+0.20 and 26.58+0.63°C showing no significant variation (ANOVA P> 0.05).
Population parameters
As for the maximum reported densities of rot . Ind-1, there were no significant differences (ANOVA P> 0.05) with the oxygen system treatment having 750+60 rot . ind-1 and control 825+33 rot . ind1. The culture declined to provide oxygen from seventh day treatment and control from the third day (Fig. 1), with significance shown for the duration of the culture (ANOVA P <0.05). In general a total of 55 × 107 rotifers were obtained in both systems.
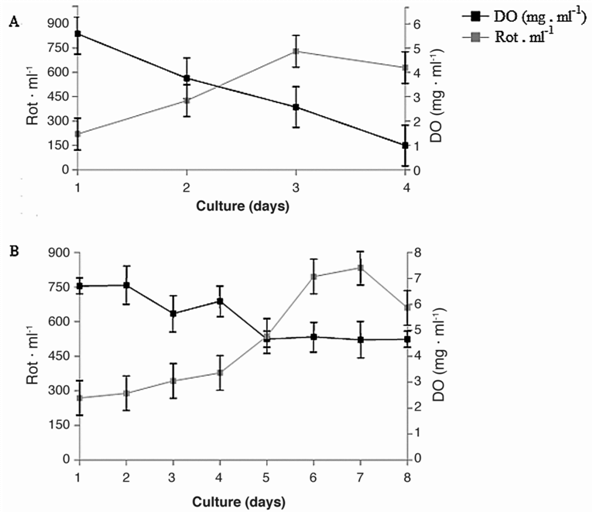
Figure 1 Density of rot . ml-1 and DO (mg . l-1) in the control culture (A) and with addition of oxygen (B).
The instantaneous growth rate, doubling time and crop yield did not differ significantly between treatments (Table 1) (ANOVA P> 0.05).
Table 1 ANOVA of instantaneous growth rate (day-1), doubling time (day-1) and population performance (rot . l-1 . day1) (mean values ± standard deviation) in both rotifer production systems.
| Variable | Treatment | P | |
|---|---|---|---|
| - | - | ||
| - | Oxigen | Without oxigen | - |
| Instantaneous growth rate. | 0,212+0,04 | 0,35+0,08 | 0,056 |
| Doubling time | 3,35+0,73 | 2,011+0,43 | 0,053 |
| Culture yield | 98.140+25,96 | 167.850+36,85 | 0,055 |
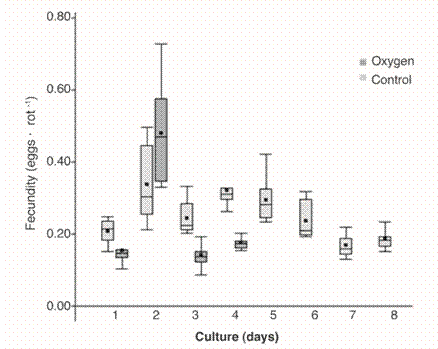
The fecundity (F) obtained from day 1 to 4 in both cultures showed statistical differences (ANOVA P <0.05), reporting for the control system the highest average for day two, and in the culture with oxygen the highest average were obtained for the remaining three days. All F exceeded 0.15 rot . l-1 . day-1 .The lowest fecundity were reported for the last days of the period of both cultures (Fig. 2).
Length and width of lorica
There were no significant differences (ANOVA p> 0.05) between the means of morphometric characteristics of both rotifers systems; the oxygen system presented an average length of 150,02+11,92 and a width of 114,10+11,92 µm, while in the control treatment a length of 147,82+11,86 and a width 111,3+11,18 µm.
Economic Analysis
It is 53% less expensive to produce rotifers without oxygen supply in the Pacific Marine Park. The 47% of total costs in the system with oxygen was used to purchase oxygen while 57% of capital invested in the control treatment was assigned to labor. In addition, a million rotifers in the culture with addition of oxygen would cost approximately $1,06 + 0,17 and in the system without oxygen addition $0,53+0,04; there is statistically significant difference between the price of a million rotifers in both systems (ANOVA P> 0.05). Table 2 shows an idea of the total costs required to develop a batch system for a month using oxygen and without oxygen in the Pacific Marine Park.
Table 2 Breakdown of Costs ($) used in the production of rotifers.
| Same costs for both rotifer production systems | ||||
|---|---|---|---|---|
| Wage | Hour cost ($) | Amount | Total ($) | |
| (hours) | ||||
| Biologist | 6,0 | 96,0 | 576,0 | |
| Assistant | 3,5 | 16,0 | 56,2 | |
| Statutory benefits | Employer contribution (%) | - | Total ($) | |
| Social chargues | 36,5 | - | 230,7 | |
| Power Consumption | Cost KWh ($) | Energy (KWh) | Total ($) | |
| - | 0,2 | - | - | |
| Air conditioning | - | 61,0 | 9,3 | |
| Algal incubator | - | 332,0 | 50,5 | |
| Autoclave | - | 172,0 | 26,2 | |
| Blower | - | 158,0 | 24,0 | |
| Microscope | - | 6,5 | 1,0 | |
| Peristaltic pumps | - | 5,4 | 0,8 | |
| Pumps | - | 8,4 | 2,5 | |
| Seawater pump | - | 8,4 | 1,3 | |
| Semi analytical balance | - | 8,0 | 1,4 | |
| Stereoscope | - | 13,5 | 2,0 | |
| Ultraviolet lamps | - | 202,0 | 30,7 | |
| TOTAL | - | - | 149,6 | |
| Reagents | Cost per gram ($) | Quantity (g) | Total ($) | |
| Ammonium chloride | 0,1 | 54,0 | 5,4 | |
| EDTA | 0,1 | 8,2 | 0,5 | |
| Ferric chloride | 0,1 | 8,7 | 0,5 | |
| Iodine | 0,1 | 10,0 | 0,8 | |
| Potassium iodide | 0,6 | 154,0 | 24,0 | |
| Sodium nitrate | 0,4 | 2,0 | 0,8 | |
| Sodium phosphate | 0,1 | 15,0 | 1,0 | |
| Vitamins | 0,0 | 30,0 | 0,3 | |
| Other specific costs for each system | ||||
| Oxygen System | Unit Cost ($) | Amount | Total ($) | |
| Oxygen | 500,0 | 2 dewar | 1.000,0 | |
| Probiotic | 1,0 | 23 pills | 23,0 | |
| Yeast | 3,8 | 13 packages | 49,4 | |
| System without oxygen | ||||
| Probiotic | 1,0 | 23 pills | 23,0 | |
| Yeast | 3,8 | 15 packages | 57,0 | |
| TOTAL OXYGEN SYSTEM | - | - | 2120,0 | |
| TOTAL SYSTEM WITHOUT OXYGEN | - | -- | 1125,8 | |
It takes $ 2 089 to feed a tank with larvae using oxygen fed produced rotifers (total cost: 70% corresponded to rotifers, 22% in microalgae, 6% for shrimp and 1.5% in otohime) and $ 1 193 with rotifers from cultures without oxygen supply (49% rotifers, 38% microalgae 10% brine and 2.5% otohime). According to these costs, a spotted snapper larvae fed with rotifers from oxygen supplied systems would cost $ 0.27 and a larva using the rotifer production control system would have an economic value of $ 0.16.
Discussion
Although the oxygen supply system sustain concentrations within the recommended by Orhum and Benetti (2001), it always declined at the population level due to the high reported values of ammonium (Yoshimura et al., 2003). The control treatment exemplified the two greatest constraints to the development of cultures exposed by Yoshimura et al. (2003) corresponding to the low concentration of DO (> 1 mg ∙ l-1) (fig.1) and high values of ionized ammonia, thus also its short duration of 4+1 days. High concentrations of nitrogen compounds can be controlled with water exchange (Orhum and Benetti, 2001) or with water recirculation systems using biofilters and ozone (Bentley, 2008).The temperatures reported in this paper are considered common to the Gulf of Nicoya (Vega 2010), and are within the recommended Orhum and Benetti (2001), who suggest that temperatures in rotifer cultures must not exceed 30°C range since it affects the levels of DO and reproductive capacity of organisms. As for pH, eventhough these organisms can survive within wide ranges (Yin and Niu, 2008), Fielder et al., (2000) recommends a range between 7.4 and 8.2, which agrees with the values obtained in this work.
Regarding the highest population densities obtained on both systems (> 600 rot∙ ml-1), these are considered common for tanks from 500 to 1000 L in batch rotifer production centers (Dhert et al., 2001). In terms of population parameters (Table 1), both growth rates are considered typical within the stated range for B. plicatilis in batch cultures (Suantika et al., 2003, Tinh et al., 2006). The doubling times of both systems match the reported (2 to 3.3 day -1) range by Vallejo et al., (1993) in his study of B. plicatilis. Population yields of both crops are considered high compared to that reported by Abu-Rezq and James (2005) of 12.130+1,89 rot ∙ l-1 ∙ day-1and that of Cisneros (2011) ranging from 1.330+0,33 a 13.580+0,02 rot ∙ l-1 ∙ day-1. The yields of rotifers improve the more HUFA’s and proteins their feeds contain (Dhert et al., 2001; Cisneros, 2011), adding to the success of using microalgae on such cultures (Suantika et al, 2003, Aragão et al., 2004, Benetti et al., 2008, Ferreira et al., 2008).
The fecundity obtained in this work are considered most suitable (<0.2) to maintain stable crops (Orhum and Benetti, 2001), however these forms decreased with increasing days in culture due to deterioration of the quality of water (Hagiwara et al., 2007).Regarding the size of the rotifer, this must be consistent with the mouth opening of the larvae which is being grown (Hagiwara et al., 2001). An amount of 50% of the lengths of the rotifers reported in this paper are on the size limit recommended by Boza et al., (2008), indicating that for the production of Lutjanus guttatus the zooplankton to be used must be than 150 microns in length. In addition, the sizes of rotifers of both systems coincide with the results obtained by Hagiwara (2001), the author reports that the size of the species Brachionus sp. are within a range of 90 and 340 µm. As there is no statistical differences between the morphometric characteristics in both crops it can be said that oxygen is not a variable that influences this aspect, the above is further supported by Hagiwara et al., (2007) where it is mentioned that the size of rotifers can be modified by sudden changes in temperature, salinity or with different food regime.
From an economic standpoint, the non-use of oxygen brings more economic benefits to Pacific Marine Park since to produce 2,000 million rotifers day-1, and costs are 60% cheaper than with oxygen supply. The reported cost for a million of rotifers in this study was higher than those published by Bentley et al., (2008)($ 0.009) and Suantika et al., (2003) ($ 0.048), however, it was lower than Alvarez-Lajonchere and Álvarez et.al, (2013) at a cost of $ 1.65 in batch cultures. Oxygen is used by many authors as a resource to support the stability of rotifer cultures (Kostopoulou et al., 2006; Sayegh et al., 2007; Papakostas et al., 2007; Kobayashi et al ., 2008; Larsen et al. 2008; Yin and Zhao, 2008; Cavalin and Weirich, 2009; Mahmoudzadeh et a.l, 2009; Qi et al, 2009), however, in the Pacific Marine Park adding oxygen posed no technical difference (ANOVA p> 0.05) compared to control culture due to several factors; the first is related to the fact that dissolved oxygen was controlled only during the day (it was not mechanized and there were workers who labored night shift) which did not allow a good control of this variable. According to Alvarez-Lajonchere and Álvarez et.al, (2013) results in rotifer cultures depend on the correct technology developed in the laboratory. Additionally, high temperatures (Yin and Zhao, 2008) and proper nutrition (Dhert et al., 2002; Cabrera, 2008; Cisneros, 2011;. Rojo-Cebrero et al, 2012) allowed the control culture to achieve similar densities (ANOVA p> 0.05) that that of the oxygen added culture. In conclusion for the Pacific Marine Park is technically and economically more feasible the non-application of pure oxygen to rotifer cultures as long as it provides daily refills seawater to its support systems (Orhum and Benetti, 2001) to avoid early falls through ionized ammonia accumulation (Yoshimura et al., 2003).