Introduction
Spodoptera frugiperda (J. E. Smith), or the fall armyworm, belongs to the order Lepidoptera and the Noctuidae family. Due to its polyphagous behavior (Murua et al. 2009), high voracity, ability to form large populations, and high dispersion rate, this species is considered a cosmopolitan pest, one of the most destructive in America (Murua et al. 2003). The fall armyworm feeds on more than 60 species of plants, especially maize, rice, sorghum, grass, cotton, peanuts, alfalfa, oats, sugar, onions, beans, potatoes, tomato, wheat, soybean, castor oil plant, sesame, melon and sunflower among others (Carroll et al. 2006; Murua et al. 2003; Zenner et al. 2007). Because it feeds on a diverse range of crops, this insect is being studied in many laboratories around the world, therefore, maintaining experimental populations and determining their life cycle are important aspects to consider when conducting research on natural enemies, resistance testing with new insecticide molecules and in the development of biotechnological research (Murua et al. 2003).
According to Villa and Catalan (2004), the determination of various larval stages is a basic issue when constructing growth prediction models. These models are valuable tools to define a strategy for the control of pests. The biotechnological potential of natural enemies is, sometimes, unpredictable or uncertain, because, in many cases, biological cycles are unknown, and determined by environmental conditions and an ideal food requirement for normal development of the host and the bio-controller life cycle. The lack of knowledge of the requirements of beneficial biological organisms limits the implementation of a massive protocol for development in laboratory conditions.
Insect larvae undergo several molts during their development as a result of hormonal changes, which produce variations in size, behavior and morphology. The term stage indicates larval forms between successive molts, where different approaches have been proposed to identify them. Most of the stages are based on the relationship between larval cuticle and the size of various parts of the body (width and/or length of head or prothoracic shield). However, it is common to find the same value for head width in different larval stages (Villa and Catalan 2004).
As reported by Molina et al. (2003), biological control using parasitoids and predators can be very effective at low cost to replace synthetic insecticides, which unbalance the ecosystem, destroy beneficial organisms, and allow, in some cases, the development of resistant pest populations. Parasitoids are being used more frequently than insect predators in biological pest control of insects worldwide (Roomi et al. 1996). The Braconidae family is one of the most important groups of parasitic insects, which include a huge number of species that in many cases are efficient enough to make a significant impact in the biological control of many plant pests (Gozuagik et al. 2008).
Apanteles, an insect that belongs to the Himenoptera order, Apocrita suborder, Ichneumonoidea superfamily, and Braconidae family, is often used as a bioagent insect (Mehlhorn 2008). Numerous species of this genus have been reported. Roomi et al. (1996) described the biology and life cycle of A. flavipes and determined the life cycle and parasitism characteristics between Braconidae and the host larvae (Bruchus chinensis L.). That research reported an effective parasitic control that reached up to 45% in field populations, and proposed that A. flavipes is a viable option to control this pest. Other species, such as A. taragamae, have been used for biological control of Maruca vitrata (Lepidoptera, Crambidae), and pest insects of cowpea (Vigna unguiculata) (Dannon et al. 2010b).
Fig (Ficus carica) is a moraceous crop that produces a syconus fruit valued by many people over the world for its nutritional qualities and flavor. Fig cultivation occurs predominantly in temperature zones such as Spain, Turkey, Portugal, Chile and Argentina. Some of the most common pests and pathogens are the fruit fly (Ceratitis capitata), fig fly (Lonchea aristella), fig flake (Lepidosaphes fici), fig cochineal (Ceroplastes rusci), and vector-borne viruses (Fig Mosaic Virus transmitted by Aceria ficus) (Flores et al. 2011). In Costa Rica, the cultivation of fig has increased, where most of the production occurs in the northern part of the Cartago province (Tierra Blanca, Prusia) (Flores et al. 2011). A major fig-damaging insect found in Costa Rica is S. frugiperda. This study was aimed to develop a model for predicting the larval stage of S. frugiperda and to determine the effectiveness of Apanteles sp. for biological control in fig.
Materials and Methods
Egg collection, establishment of a breeding stock and identification of the Lepidopteran
Collection
Egg masses located in the abaxial side of fig leaves were collected in a plantation located in Llano Grande, Cartago at 09°57'2.91'' (± 2) north latitude and 083°55'9.01'' (± 2) west longitude at 2188 meters above sea level, and in the Campo Frutícola of the Instituto Tecnólogico de Costa Rica (ITCR) located in Los Angeles, Cartago at 09°51'09.1" (± 3) north latitude and 083°54'25.4'' (± 3) west longitude at 1405 meters above sea level. Egg masses were transferred to the Biocontrol Laboratory of the Centro de Investigación en Biotecnología (CIB) of ITCR and placed in transparent plastic containers with a volume of 60 ml under controlled environmental conditions (climate chamber) with a temperature of 24 ± 0,1 °C, 70 ± 0.5% relative humidity, and a photoperiod of 16 hours light and 8 hours dark.
Breeding stock and life cycle
After eggs have hatched, larvae were individually isolated in transparent plastic containers (volume: 60 ml), fed an artificial diet BIO-modified H-89 MIX (Doreste and Navarro 1982), and maintained in controlled environmental conditions as described above.
In the pupa stage, the larvae were kept in 500 ml, transparent plastic containers maintaining the same controlled environmental conditions. When adults emerged, 10 individuals were placed in paper bags (4000 cm3), labeled and set in the same controlled conditions to promote mating. A cotton boll impregnated with a 1:1 ratio of honey and water was placed in each bag, replacing the nectar for feeding adults. After 24 hours, the bags were opened, adults were removed and placed in a new bag, following the same procedure described above. Egg masses produced by the insects were removed and placed in clear 60 ml plastic containers until the emergence of the first instar larvae. This activity was repeated daily until all adults died. Once the lepidopteran breeding stock was established, adult insects were taxonomically identified at the Instituto Nacional de Biodiversidad (INBio) in Costa Rica.
Determination of fertility and hatchability of the Lepidopteran
Determination of fertility
After completing the lepidopteran life cycle, one male and one female were placed in a paper bag in the same controlled environmental conditions as described above, repeating this procedure until 30 couples were reached. For each mating, the number of eggs produced and the number of eggs hatched were counted. The time that females lasted in making the first oviposition and the time it took for the first hatching was recorded. Descriptive statistics of the studied variables were determined for fertility and hatchability of 30 mating couples.
A Spearman linear correlation analysis (a = 0.05) was calculated using the number of eggs per pair, number of hatched larvae per couple, days after the first oviposition and days after the first hatching, to determine important trends regarding the fertility of the species. Analysis was carried out using the statistical program Minitab(r) 16.1.0.0.
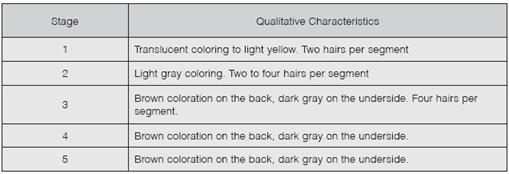
Because each larval stage presented different characteristics, the presence of hair per segment and coloring was determined for each stage. Due to the distribution of the data, a Kruskal-Wallis test (a = 0.05) was applied to determine statistical differences between the medians of the phases in regards to size. Medians were contrasted using the Mann-Whitney U test (a = 0.05). We also carried out a test of equal variances.
Development of a larval phase prediction model
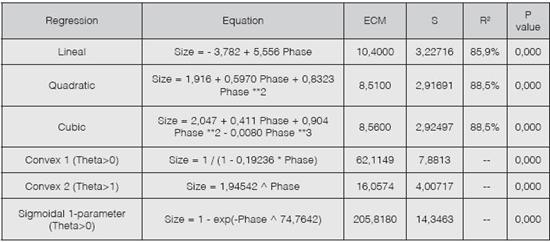
A system of linear equations (linear, quadratic and cubic) and nonlinear (convex and sigmoidal) regressions were evaluated to establish a phase prediction model related to larval size assessed by the criterion of the lowest mean square error (MSE) and the lower variance (S), using the statistical software Minitab(r) 16.1.0.0.
Isolation and Identification of a Parasitoid
From the collections made, two larvae were selected from fig plants, located in the Campo Fruticola, with symptoms of parasitism. Larvea were manipulated using the same methodology for the establishment of the Lepidoptera breeding stock, and parasitic behavior and progress was observed. Five days after larval death, an opening was produced exposing gregarious pupae of the endoparasitoid insect, which were then placed in 500 ml plastic containers, keeping the same controlled environmental conditions described above until the adults emerged.
Adult parasitoids were kept in plastic containers and fed with honey and water in a 1:1 ratio that was applied in drops over the entire surface of the container. Three adult parasitoids were selected and then identified by specialists at INBio of Costa Rica.
Results
Egg collection, establishment of a breeding stock and identification of the Lepidopteran.
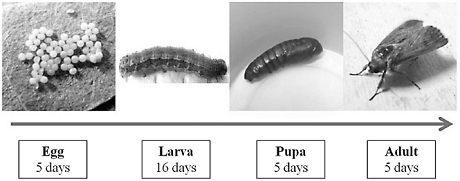
Six hundred twenty three larvae were isolated from the egg masses collected from the abaxial side of the leaves. A survival rate of 31.13% was recorded from a total of 194 larvae. The Lepidopteran life cycle took on average of 38 days to complete in controlled environmental conditions (Fig 1). According to the taxonomic identification done by the specialist Jose Montero, the lepidopteran was identified as S. frugiperda.
Determination of fertility and hatchability of the Lepidopteran
Table 1 shows the average, standard deviation and Spearman correlation for the studied variables. It was determined that, on average, S. frugiperda larvae oviposits 502.53 eggs at about 2.88 days after mating, of which 303.75 are fertile and hatch about 6.13 days after being laid. Furthermore, we observed that for every 10 eggs laid per pair, 6.27 larvae hatched. For the other correlations performed, there is insufficient statistical evidence of correlation.
Based on the qualitative characteristics (Table 2) and larvae size (Fig. 2), it was determined that the insect presented five larval stages, where variability of insect size increases as larvae develop.
Statistical significant differences between each of the larval stages were determined for the mean and the median of the larvae size according to the stage. Furthermore, it was determined that as the larval stage increases, the size variability, the standard deviation, and the amplitude of the stage also increase (Fig. 2).
Table 1: Spearman correlation (a=0, 05) for studied variables of fertility and hatchability in Spodoptera frugiperda.
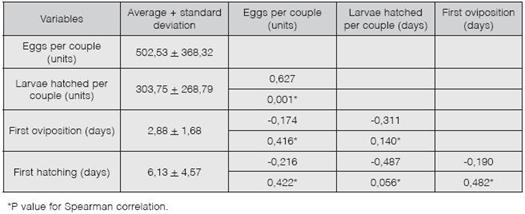
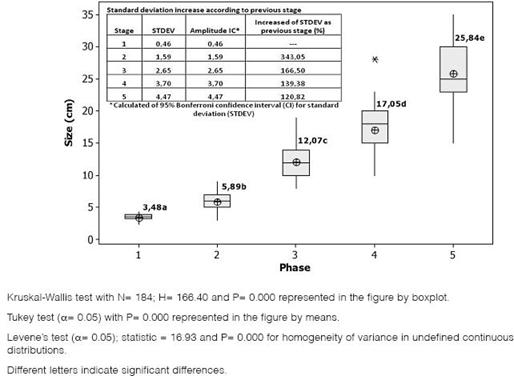
Figure 2: Equal variances, amplitude and size variability in each larval stage of Spodoptera frugiperda.
Different regression models, prediction equations, and P values per each evaluated regression were determined (Table 3). A quadratic regression is the best fit to the model, due to its lower mean square error and standard deviation with respect to the other regressions.
Isolation and Identification of a Parasitoid
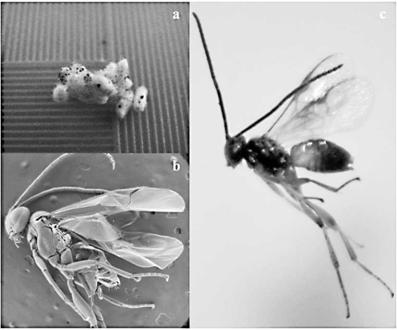
Pupae and adult parasitoid collected from S. frugiperda larvae are shown in Fig 3. The parasitoid was identified taxonomically as Apanteles sp.
Discussion
Egg collection, establishment of a breeding stock and insect identification
Murua et al. (2009) determined the temporal and spatial distribution of larval populations of S. frugiperda in different hosts in Argentina. During three years of study, they found larvae in corn, grain sorghum, alfalfa and weeds. In grasses, such as corn and sorghum, the presence of the pest is considered endemic, which means there are always pest populations that cause damage to the crop in varying proportion. In other plants, the attack seems to be unpredictable. There are seasons where insect populations are not important, while other seasons require continuous monitoring, often even without success by the presence of high populations and tolerance or insect resistance to the chemicals used.
Casmuz et al. (2010) conducted a review of the armyworm hosts in America, finding a total of 186 hosts distributed in 42 families; Poaceae and Fabaceae families are among the most cited. The authors present only one report for the genus Ficus (commonly called rubber tree), however in that study, they found the incidence of S. frugiperda attacking fig (Ficus carica) in the northern part of Cartago, Costa Rica.
For the establishment of breeding stock, survival was about 31.13% of eggs collected, however these values do not match those found by Chacon et al. (2009), where 45 larvae were collected then introduced to the laboratory under controlled environmental conditions, and obtained a survival rate of 64.44%. Decreased survival rate obtained in this study could be due to the introduction of S. frugiperda eggs into the laboratory, not larvae, and the shift from uncontrolled climatic conditions to controlled environmental conditions.
For the establishment of breeding stock of S. frugiperda larvae, individual collection is recommended, because according to Chapman et al. (2000), many species of Lepidoptera larvae often practice cannibalism as a taxonomically widespread behavior, influencing population dynamics and community structure. Cannibalism is a common behavior in fall armyworms reared in laboratory, even when food is not a limiting factor, and can cause 40% to 60% of deaths.
In the study herein, the life cycle of S. frugiperda was determined in 38 days under light and temperature controlled conditions and using the modified BIO-MIX H-89 artificial diet (Navarro and Doreste 1982). However, Chacon et al. (2009) determined the armyworm life cycle in 47.40 ± 1.60 days under controlled environmental conditions using a diet of young corn leaves, but when using BIO-MIX H-89 artificial diet, the life cycle obtained was about 45.10 ± 1.20 days. According to Murua et al. (2003), using artificial diets provide a complete food source easy to use, as long as all the necessary elements to enable the development are provided. These researchers concluded that diet has a significant influence on duration and mortality of individuals throughout the life cycle of S. frugiperda, and that an artificially-based diet on white bean flour, wheat germ, brewer's yeast powder, agar, ascorbic acid, sorbic acid, nipagin, formaldehyde and distilled water was the best diet evaluated because of the proportion of individuals who reached adulthood, the duration of the cycle life and the life expectancy average of individuals.
Nutritional factors affect physiology, behavior, ecology and evolution of insect species. The quality and quantity of consumed food during the larval phase affects the growth rate, development time, body weight and survival. This phenomenon also affects fertility and longevity of insect adults (Santos et al. 2003), and for that reason we worked with an artificial diet develop by Navarro and Doreste (1982), which contains known homogeneous nutritional values so they can directly influence shortening of the life cycle of S. frugiperda.
Temperature is another strong factor in the biology of the species, as mentioned by Clavijo et al. (1991). In the case of S. frugiperda, as most of the lepidopeteran species, when temperature increased, the duration of each phase decreased, showing a life cycle of 26.8 days at 35.3 °C, 43.5 days at 25.0 °C, and larval death at 15.3 °C. In this study, a life cycle of 38 days was obtained under controlled temperature, humidity and a suitable artificial feed. Clavijo et al. (1991) concluded that although S. frugiperda is capable of withstanding constant temperatures within a fairly wide range, between 15 °C to 35 °C, its development, mortality and reproductive capacity are favored at temperatures ranging between 20 °C and 30 °C, with an average of 25 °C. Furthermore, Heinrichs et al. (2000) found that embryonic development of the armyworm takes 6 days at 20°C and 2 days at 30°C due to lower temperatures which favor enzyme activity reduction during physiological processes of insects, whereas high temperatures enhance metabolic activity, reducing life cycle time, as long as there are no stress caused by environmental or food factors.
Determination of fertility and egg hatchability
The average number of S. frugiperda eggs was 502.53 eggs per pair, with a fertility percentage of 60.44%. These data are consistent with those obtained by Clavijo et al. (1991) where a fertility value of 635.2 eggs was obtained; a fertility percentage of 64.9% at 25 °C. However, S. frugiperda females lay up 200 eggs at a time, often exceeding the food resource capacity for the survival of their offspring (Carroll et al. 2006). Important related studies have been performed, obtaining contrasting values with each other, which depend on temperature, humidity, and the armyworm isolation place (Navarro and Doreste, 1982; Clavijo et al, 1991; Chacon et al, 2009).
The Spearman correlation (0.627) indicated that there is a positive correlation between the number of eggs per pair in relation with the number of hatched eggs, where for every 10 laid eggs, 6.27 larvae hatched, which is consistent with the obtained fertility percentage.
Regarding larval stages, S. frugiperda usually has six larval instars (Villa and Catalan 2004), however these could vary according to the supplying diet. Murua et al. (2003) evaluated the number and duration of S. frugiperda larval stages under different artificial diets based on white bean flour, corn flour, white corn pieces and rice, varying from six to eight larval stages and then determined a high larvae mortality using an artificial diet based on corn flour.
In another study by Santos et al. (2003), larval and pupal stages were determined at different maize genotypes, finding from 5 to 7 larval stages without differences related with maize genotype, where the fifth stage predominated. Similarly, Villa and Catalan (2004) established a breeding stock to develop a predictive model of S. frugiperda, however only 18% of larvea were able to reach the sixth stage, an the fifth stage was the most frequently obtained in laboratory. This study determined five larval instars using an artificial diet under controlled environmental conditions.
Santos et al. (2003) performed a regression analysis based on the S. frugiperda head capsule, where they determined that there were no significant differences between the first, second and third larval instar. The model presented was a linear regression of the stage over the natural logarithm of the head capsule size. On the other hand, Villa and Catalan (2004) developed a prediction model based on larval size and head width, demonstrating that as the insect grows there is greater variability of the head width. However, the head capsule size and the width of the head of the S. frugiperda larvae often have the same values for different stages, for this reason, in this study the prediction model was determined based only on the larval size.
To perform a prediction model, a Kruskal-Wallis variance analysis was made first, a Mann- Whitney test, and equality of variances. There were significant differences between all treatments, a result that is not consistent with that obtained by Santos et al. (2003) and Villa and Catalan (2004), as they found no significant differences in the early stages. Furthermore, it was determined that increasing the larval stage increases data amplitude and standard deviation, this could be because as larvae develop, other factors influence S. frugiperda growth such as genetic factors, adaptation to controlled environmental conditions, and the intrinsic biological behavior.
The proposed equation that predicts the size of the larval stage is quadratic and is expressed as:
Size = 1.916 + 0.5970 Phase + 0.8323 Phase2
which was chosen because it had a large R2 value, the lowest deviation and the least mean square error of all regressions. Moreover, qualitative characteristics like color and the number of hairs per segment provides a qualitative description that is complementary with the prediction model. Regarding pupal color, it was determined that white indicates an obteca immature pupa and that mahogany is an obteca mature pupa.
Isolation and identification of a Parasitoid
Different species of Apanteles have been used for biological pest control (Roomi et al. 1996; Takacs et al. 1997; Whitfield et al. 2001; Sertkaya et al. 2004; and Dannon et al. 2010b). Potential agents of biological control, predators and parasitoids are classified as the most effective. Hymenoptera and Diptera are the orders that have the highest number of armyworm parasitoid species where there are reports of Apanteles congregata, A. marginiventris and A. ruficurs (Molina et al. 2003). As mentioned by Vilaseca et al. (2008), species that have been recognized with higher parasitism over S. frugiperda are Lespesia sp., Archytas mormoratus, Apanteles marginiventris, Campoletis sp. Chelonus insularis, Ophion sp., and Meteorus laphygmae.
Vilaseca et al. (2008) determined a parasitism about 24.8% associated with rice and palm cultivations and about 28.1% associated with rice and forest with A. marginiventris. This species is an important parasitoid in the natural biological control of S. frugiperda larvae, because this parasitoid is characterized by its ability to attack armyworm populations when pest densities are low. Vilaseca el al. (2008) also mention that this species has a high capacity for movement and colonization. This study reports the discovery of Apanteles sp. as a parasitoid of S. frugiperda in fig cultivation in Costa Rica.
Conclusions
Under controlled environmental conditions, i.e. 24°C, 70% relative humidity and an artificial diet, the life cycle of S. frugiperda lasts 38 days in total. A Spearman correlation was established between the number of eggs produced per pair and the number of hatched larvae. A statistically significant quadratic equation was developed to infer larval stage based on size of each stage. Finally, it is reported that S. frugiperda is an insect that attacks fig cultivations in Costa Rica and Apanteles sp. is a possible biological controller of fall armyworm.