Introduction
Mosquitoes (Diptera: Culicidae) are vectors of pathogens that include viruses (arboviruses), filarial worms (helminths) and protozoa that are significant causes of morbidity and mortality worldwide, particularly in tropical and subtropical countries (Bueno-Marí et al., 2015). During the COVID-19 pandemic, the significant surge in dengue cases across the Americas emphasized the critical role of mosquitoes in public health (OPS/OMS, 2020).
Culicidae is diverse, currently with 3 718 species classified into two subfamilies. Anophelinae comprised of three genera, Culicinae divided into 11 tribes, encompassing 113 genera (Harbach & Wilkerson, 2023). In Ecuador, there are recorded species from both subfamilies, eight tribes, 22 genera, totaling 200 species (Ponce et al., 2021; Ramón et al., 2019).
Although vector-borne diseases are prevalent in the Amazonian and Coastal regions of Ecuador, there is a scarcity of ecological studies focused on Culicidae, and the majority of the existing research is relatively recent (Duque et al., 2019; Navarro et al., 2015). According to the Ministerio de Salud Pública del Ecuador (2020), Ministerio de Salud Pública del Ecuador (2021) and Ministerio de Salud Pública del Ecuador (2022), Ecuador has repofigrted cases of dengue, Zika, chikungunya, Mayaro, yellow fever, and malaria. The number of dengue cases has shown an upward trend, with 8 416 cases reported in 2019, followed by 16 570 and 20 593 cases in 2020 and 2021, respectively. In terms of dengue incidence, Napo province ranked third in the Ecuadorian Amazon region in 2019. However, there was a surge in cases in 2021, surpassing all other provinces and recording the highest number of dengue cases that year. While malaria cases have also witnessed an increase, they remain primarily concentrated in other provinces within the Amazonian region.
Community and adventure tourism in Napo has increased over the years, because of the many existing attractions, high biodiversity, and extensive protected areas. La Isla Amazon Park (PALI, called after its Spanish initials) is an urban and touristic green area for recreational purposes and serves as a habitat for various vertebrate species. PALI is essential for tourist and ecological interests, as well as for public health because a large flow of people and the presence of possible vectors increases the probability and risk of disease transmission (Duque et al., 2019).
Effective understanding and prevention of arthropod-borne diseases hinges upon a comprehensive understanding of species composition, distribution, and ecology (Ceretti-Junior et al., 2016). This study aimed to document the Culicidae species found in La Isla Amazon Park (PALI), Napo, Ecuador, including those with epidemiological significance; and to analyze their composition, abundance, and diversity, focusing on larval habitats during dry and rainy periods.
Materials and methods
Study site: The confluence of the Pano and Tena rivers forms a peninsula known as PALI in Tena, Napo province (0°59’44.45’’ S & 77°49’7.31’’ W; 0°59’49.39’’ S & 77°49’2.053’’ W; 0°59’49.27’’ S & 77°49’5.92’’ W; 0°59’34.52’’ S & 77°48’55.92’’ W). The Colonso Chalupas Biological Reserve (RBCC), created in 2014, is the closest protected area to PALI (Fig. 1). PALI spans 24 hectares, featuring a native species forest and a natural wetland; eight hectares are designated for tourism, including four kilometers of trails used in this study. Despite multiple floods over the years, the park continues to be inhabited by many species that were donated to it in 1995, when it was opened. The park is in a humid, tropical zone with abundant bimodal rainfall (peaks tend to occur in June and November), high relative humidity (> 85 %), and 22.8 °C mean annual temperature (Lucas-Solis et al., 2021).

Fig. 1 Location map showing PALI sampling patches and relative position in Napo, Ecuador. Empty pins indicate the dry period, and filled pins indicate the rainy period.
Ethics Statement: The authorization for the Collection of Specimens of Biological Diversity for Non-Commercial Purposes No. 1176 (MAAE-ARSFC-2021-1176) issued by the Ministerio del Ambiente, Agua y Transición Ecológica of Ecuador was processed.
The collected specimens were deposited in the invertebrate wet section of the Museo de Ciencias Naturalesat the Instituto Nacional de Biodiversidad (INABIO), from catalog number MECN-MA-8603 to MECN-MA-8620.
Culicidae collection: Collections of mosquitoes were carried out for immature and adult stages during a rainy (26-28/07/2021) and dry (29-31/03/2021) period. Immature sampling points were plotted over the area designated for tourism development. Sampling was standardized by using collection duration as the main criterion (30 min per patch) and the number of sampling points was determined by the availability of breeding sites along the trail. Immatures were sampled in phytotelmata, as well as in ponds and artificial containers. For large water bodies, the systematic scooping technique was used; the pond margin was subdivided into subsampling points. In other words, the 30 min were divided by 5 min, which means there were 6 subsampling points.
Thus, distance between points depended on the diameter of the pond (Urbinatti et al., 2001). The specimens were collected using a suction pipette or dipper, depending on the type of larval habitat, and temporarily stored in sterilized sampling bags. Some immatures were deposited in individual breeding vials for development, and the remainder preserved in cryovials with a 10 % formaldehyde solution. The collected specimens were deposited in the invertebrate wet section of the Museo de Ciencias Naturales at the Instituto Nacional de Biodiversidad (INABIO), from catalog number MECN-MA-8603 to MECN-MA-8620.
Traps baited with dry ice (CO2) and UV light were used to sample adult mosquitoes: CDC trap and a modified Shannon trap, which do not use animals as bait (Silver, 2008). The CDC trap remained active for 14 h (18:00-08:00) and Shannon traps remained active for 4 h (18:00-22:00). Periodic trap sampling was carried out during that time, checking the outside and inside of the trap every 15 min for 5 min. Mosquitoes were collected using an insect aspirator, sacrificed through low-temperature exposure, mounted on entomological pins, and stored in entomological boxes treated with naphthalene and silica gel.
An AmScope trinocular stereo microscope with white light and 90X magnification was used for species recognition based on available keys and taxonomic revisions (Forattini, 1965; González & Darsie Jr., 1996; Lane, 1953a; Lane, 1953b; Liria & Navarro, 2003; Sallum Mureb & Forattini, 1996; Valencia, 1973; Zavortink, 1972), and the interactive identification keys of Walter Reed Biosystematics Unit (2021). The genera and subgenera abbreviations follow Wilkerson et al. (2015).
Data analysis: Sampling effort was assessed using species accumulation curves and nonparametric estimators of species richness: Abundance-based Coverage Estimator (ACE), Chao1, Jackknife 1, and Bootstrap (Colwell et al., 2004). Randomizations (1 000) without replacement and a 95 % confidence interval were used. The upper limit of abundance for rare or infrequent species used in the ACE statistic was 7, based on the categories proposed by Friebe according to Lira-Vieira et al. (2013). It is considered rare when D < 1 %, whereas D % = (total number of individuals of the species / total number of individuals captured) * 100.
Diversity was estimated through Hill numbers (Jost & González-Oreja, 2012), where q is the order of diversity (qD) and determines how influential common species or rare species are in the measurement. The first three Hill numbers, q = 0 (richness), q = 1 (Shannon’s exponential diversity), and q = 2 (Simpson’s inverse diversity), were assessed to measure the effective number of species, an equivalent measure of true diversity.
The Berger-Parker index and the Pielou index were calculated to quantify the species dominance and evenness component between seasonal periods. The similarity between habitats was assessed with a cluster analysis (UPGMA) dendrogram, using the Bray-Curtis distance matrix.
The differences in taxonomic composition were assessed using the analysis of similarity (ANOSIM) test with the Bray-Curtis distance and 1 000 permutations, performing pairwise comparisons using the Bonferroni correction. ANOSIM is based on comparing distances between groups with distances within groups, and the resulting R-test statistic measures whether there is a separation of community structure, and the p-value determines significant comparisons (Clarke, 1993).
The statistics were run in EstimateS Program version 9.1.0 (Colwell, 2022), PAST version 4.09 (Hammer, 2022), and Microsoft Excel (Microsoft Corporation, 2016).
Results
In total, 802 specimens (immatures and adults) were collected, identified, and grouped into 15 species and 3 taxonomic units: 10 subgenera, 5 genera, 4 tribes (Culicini: Culex, Sabethini: Limatus and Wyeomyia, Aedini: Psorophora, Toxorhynchitini: Toxorhynchites) from 7 types of larval habitats (Table 1, Table 2). During the dry period, 7 species and 3 taxonomic units were recorded, compared to 13 species and 2 taxonomic units for the rainy period.
Table 1 Abundance and diversity of immature species by habitat and collection period.
| Habitat | Speciesa | Abundance | Sb | exp(H)c | 1/Dd | de | Jf |
| DRY PERIOD | 253 | 7 | 4 | 3 | 0.48 | 0.69 | |
| Artificial | Culex (Car.) urichii | 24 | 3 | 2 | 2 | 0.59 | 0.82 |
| Limatus durhamii | 13 | ||||||
| Limatus sp. 1 | 4 | ||||||
| Bamboo | Culex (Car.) secundus | 4 | 3 | 2 | 2 | 0.65 | 0.81 |
| Culex (Car.) urichii | 15 | ||||||
| Wyeomyia (Wyo.) melanopus | 4 | ||||||
| Bromeliad | Wyeomyia (Wyo.) medioalbipes | 67 | 1 | 1 | 1 | 1 | - |
| Calathea | Wyeomyia (Dec.) ulocoma | 27 | 1 | 1 | 1 | 1 | - |
| Heliconia | Wyeomyia (Dec.) ulocoma | 95 | 1 | 1 | 1 | 1 | - |
| RAINY PERIOD | 488 | 11 | 2 | 1 | 0.81 | 0.35 | |
| Artificial | Culex (Car.) urichii | 8 | 1 | 1 | 1 | 1 | - |
| Bamboo | Culex (Car.) secundus | 18 | 4 | 3 | 2 | 0.48 | 0.69 |
| Culex (Car.) urichii | 19 | ||||||
| Culex (Cux.) dolosus | 2 | ||||||
| Toxorhynchites (Lyn.) theobaldi | 1 | ||||||
| Bromeliad | Culex (Mcx.) imitator | 1 | 2 | 1 | 1 | 0.93 | 0.35 |
| Wyeomyia (Wyo.) medioalbipes | 14 | ||||||
| Heliconia | Wyeomyia (Dec.) felicia | 12 | 2 | 1 | 1 | 0.97 | 0.19 |
| Wyeomyia (Dec.) ulocoma | 397 | ||||||
| Pond | Culex (Mel.) dunni | 9 | 2 | 2 | 1 | 0.82 | 0.68 |
| Culex (Ncx.) derivator | 2 | ||||||
| Tree hole | Wyeomyia (Wyo.) celaenocephala | 5 | 1 | 1 | 1 | 1 | - |
a: subgenera: (Car.) Carrollia Lutz, (Wyo.) Wyeomyia Theobald, (Dec.) Decamyia Dyar, (Ncx.) Neoculex Dyar, (Cux.) Culex Linnaeus, (Mel.) Melanoconion Theobald, (Mcx.) Microculex Theobald, (Lyn.) Lynchiella Lahille. b: richness (0D); c: Shannon diversity index (1D); d: Simpson’s diversity index (2D); e: Berger-Parker dominance index; f: Pielou’s equality index.
Table 2 Abundance and diversity of adult species by trap and collection period.
| Trap | Speciesa | Abundance | Sb | exp(H)c | 1/Dd | de | Jf |
| DRY PERIOD | 12 | 4 | 4 | 3 | 0.42 | 0.91 | |
| CDC | Culex (Cux.) sp. 1 | 2 | 3 | 3 | 3 | 0.4 | 0.96 |
| Wyeomyia (Dec.) ulocoma | 1 | ||||||
| Psorophora (Gra.) dimidiata | 2 | ||||||
| Shannon | Culex (Cux.) sp. 1 | 1 | 3 | 3 | 3 | 0.43 | 0.91 |
| Culex (Cux.) sp. 2 | 3 | ||||||
| Psorophora (Gra.) dimidiata | 3 | ||||||
| RAINY PERIOD | 49 | 5 | 3 | 2 | 0.61 | 0.73 | |
| CDC | Culex (Mel.) dunni | 3 | 5 | 4 | 4 | 0.4 | 0.91 |
| Culex (Cux.) sp. 2 | 3 | ||||||
| Culex (Cux.) sp. 3 | 1 | ||||||
| Psorophora (Gra.) dimidiata | 6 | ||||||
| Psorophora (Jan.) ferox | 2 | ||||||
| Shannon | Culex (Mel.) dunni | 4 | 4 | 2 | 2 | 0.71 | 0.66 |
| Culex (Cux.) sp. 3 | 2 | ||||||
| Psorophora (Gra.) dimidiata | 24 | ||||||
| Psorophora (Jan.) ferox | 4 | ||||||
a: subgenera: (Dec.) Decamyia Dyar, (Cux.) Culex Linnaeus, (Mel.) Melanoconion Theobald, (Gra.) Grabhamia Theobald, (Jan.) Janthinosoma Lynch Arribálzaga. b: richness (0D); c: Shannon diversity index (1D); d: Simpson’s diversity index (2D); e: Berger-Parker dominance index; f: Pielou’s equality index.
Wyeomyia ulocoma (Theobald, 1903) was the most abundant immature species, with 48 % of the total abundance recorded for the dry period and 81 % in the rainy period, followed by Wy. medioalbipes (Lutz, 1904) (28 %) during the dry period and Culex urichii (Coquillett, 1906) (6 %) during the rainy period.
Both periods evaluated 12 breeding sites corresponding to 7 types. Both periods have four types of immature habitats (artificial, bromeliad, bamboo, and Heliconia spp.) in common. However, Calathea sp. (near Calathea crotalifera) as a habitat was only reported during the dry period, and tree holes and ponds were only reported during the rainy period.
From the CDC and Shannon traps, 61 adult mosquitoes of 4 species and 3 taxonomic units, 4 subgenera, 3 genera, and 3 tribes were collected (Table 2). The Shannon trap was more efficient than the CDC trap in recording abundance during the dry (58 %) and rainy (69 %) periods. Psorophora dimidiata (Cerqueira, 1943) was the most abundant adult species, with 42 % of total abundance recorded for the dry period and 61 % in the rainy period.
The diversity of the dry period in both immature and adult stage, when considering all species with a weight proportional to their abundance (Hill number 1) and giving more weight to dominant species (Hill number 2) showed a slight increase with four equally common species and three effective species, respectably (Table 1, Table 2). During the rainy period, the Berger-Parker and Pielou indices indicate that there was a greater dominance of certain species and less uniformity in the abundance of species, corroborating that rare species were mainly recorded during this period.
Diversity by larval habitat showed bamboo as the most diverse of natural origin in both periods. Although artificial and bamboo exhibited the same diversity during the dry period, bamboo showed higher diversity and uniformity in the data (Table 1). The CDC trap exhibited the highest diversity during the rainy season. However, both traps displayed similar diversity levels during the dry period (Table 2).
According to the rarefaction curves sampling sufficiency was not reached since the cumulative number of species did not reach an asymptote at the end of both collection periods (Fig. 2). However, ACE and Chao 1 non-parametric estimators reached 100 % coverage in the dry period (Table 2), and Chao 1 reached 100 % coverage during the rainy period (Table 3). Although species richness was higher during the rainy period, confidence intervals of both periods are overlapping, meaning they do not differ significantly (Colwell et al., 2004).
Non-parametric estimators suggested that the estimated richness was: 8.01 ± 1.31 species for the dry period and 13.87 ± 2.57 species for the rainy period. The sampling effort recorded most of the richness since sampling completeness exceeded 75 % (Table 3) in both periods.
Table 3 Observed and expected number of immature species from both periods.
| Non-parametric estimators of richness | Periods | |
| Dry | Rainy | |
| ACE | 7 | 13 |
| Chao1 | 7 | 11.33 |
| Jack1 | 9.75 | 17.42 |
| Bootstrap | 8.3 | 13.73 |
| Observed species | 7 | 11 |
| Meana | 8.01 | 13.87 |
| SDb | 1.31 | 2.57 |
| %c | 87.36 | 79.31 |
a: mean of the estimated richness among the 4 non-parametric estimators; b: standard deviation; c: measure of sampling completeness = observed species divided / mean of the estimators * 100.
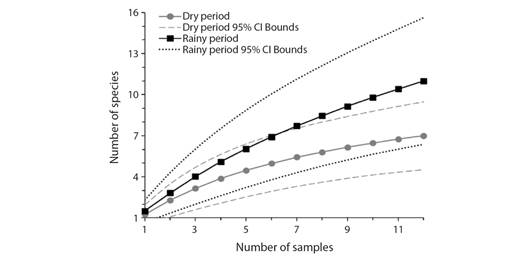
Fig. 2 Accumulation curves of immatures per sample. Dashed lines show their confidence interval bounds for each period.
Cluster analysis produced well-supported branches with a high cophenetic correlation of 0.99 (Fig. 3). Two groups of larval habitats were formed based on their taxonomic composition: 1) Calathea sp. and Heliconia spp. inflorescences, 2) artificial and Bamboo cavities.
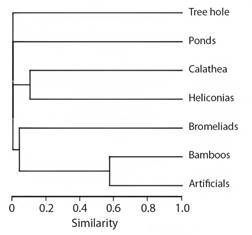
Fig. 3 Cluster analysis (UPGMA) dendrogram of larval habitats in the dry and rainy periods, based on the Bray-Curtis distance matrix.
ANOSIM indicated no significant differences in taxonomic composition of the breeding sites between the dry and rainy periods (R = -0.115, P > 0.05). The negative value of R suggested greater dissimilarity within periods than between them (Chapman & Underwood, 1999). In other words, the taxonomic composition differs between larval habitat types rather than between periods.
Discussion
No previous studies have been conducted on mosquito diversity and distribution of breeding sites in urban parks in Ecuador. PALI shares a similar species richness to Chico Mendes Ecological Park and Carmo Park in São Paulo, Brazil (Bicudo de Paula et al., 2015). The three parks are similar in size, but the parks in Brazil had a higher abundance of individuals due to the longer collection period and greater availability of breeding sites. This highlighted the richness found in PALI since a similar number of species were found for fewer individuals sampled.
During the rainy period almost twice as many immature individuals and a slightly higher species richness were recorded, although not enough to be significantly different from the dry period. Nevertheless, diversity was slightly higher during the dry period due to the disproportionate increase of Wy. ulocoma immatures and Ps. dimidiata adults during the rainy period.
Although ACE and Chao1 estimators resulted in 100 % sampling completeness, and the mean sampling completeness exceeded 75 %, the rarefaction curves did not reach sampling sufficiency. The literature states that in invertebrate studies, such as arthropods, mainly in tropical areas, asymptotes may never be reached because these are taxon-rich groups (Gotelli & Colwell, 2001).
The Calathea sp. and tree hole patch occurred once. Consequently, Wy. celaenocephala (Dyar & Knab, 1906) was only found in that single patch. For aesthetic reasons, the park is constantly being landscaped, cleared, and cleaned; therefore, the Calathea sp. patch was removed, and the bromeliad patches were reduced after the dry period. In other words, there is a strong human influence on the environment, which certainly modifies the distribution patterns of species. And, regarding the availability of breeding sites between periods, it is premature to conclude that it was the same since anthropogenic activity played a significant role. However, the occurrence of the tree hole patch during the rainy season may be due to a meteorological factor.
The species in this study were mostly specialized in a specific larval habitat type, except for Wy. Ulocoma and Cx. urichii. In fact, the cluster analysis revealed two groups with shared species: 1) inflorescences of Calathea sp. and Heliconia spp. with Wy. ulocoma, although the disproportionate abundance of Wy. ulocoma in Heliconia generated low similarity; 2) artificial and Bamboo cavities with Cx. urichii. However, the tree hole, ponds, and bromeliads had distinct compositions and did not colonize other habitat types.
Wyeomyia ulocoma, Wy. medioalbipes, Wyeomyia felicia (Dyar & Nuñez Tovar, 1927), Culex imitator (Theobald, 1903), Culex dolosus (Lynch Arribálzaga, 1891), Culex secundus (Bonne-Wepster & Bonne, 1920) were found as established in the literature (Chaverri et al., 2018; Frank & Curtis, 1981; Lane & Cerqueira, 1942; Medeiros-Sousa et al., 2015; Navarro et al., 2007; Valencia, 1973).
Wyeomyia celaenocephala was found in a tree hole, which has not been described as a breeding site for this species in particular, but for its subgenus (Frank & Curtis, 1981; Lane & Cerqueira, 1942; Navarro et al., 2015). Likewise, Wyeomyia melanopus (Dyar, 1919) was found in bamboo stumps, a larval habitat not previously reported for this species, although it has been for other members of the subgenus (Lane, 1953b; Lane & Cerqueira, 1942).
Culex urichii was found in bamboo stumps and artificial containers. This aligns with literature describing the species in a wide variety of larval habitats (Feijó Almeida et al., 2020; Liria & Navarro, 2003). Records of Cx. urichii have been found in the husks of white cacao (Theobroma grandiflorum) (Berti et al., 2013) and bacao (Theobroma bicolor) (Booth, 2018). While both Theobroma species have been described in Ecuador (Duque et al., 2019; Ponce et al., 2021), there are no records linking them to Culicidae.
Culex dunni (Dyar, 1918) and Cx. derivator (Dyar & Knab, 1906) were only found during the rainy period in the “moretal”, a swampy and permanently flooded area typical of the Amazon region, characterized by the predominant growth of the morete palm tree (Mauritia flexuosa). Supporting the description of Cx. dunni being collected from natural ponds (Bangher, 2020), and subgenus Neoculex inhabiting groundwater bodies (Harbach, 2009).
Toxorhynchites theobaldi (Dyar & Knab,1906) was found in a bamboo stump, indicating its presence in natural larval habitats. However, it has also been documented in tires, plastic, and glass containers (Ceretti-Junior et al., 2016). Toxorhynchites is a genus of great interest in biological control studies, because are predators of other immature species, and as adults are not hematophagous.
Limatus durhamii (Theobald, 1901) was found in artificial containers, but there are records of the species in natural breeding sites as well (Chaverri et al., 2018; Feijó Almeida et al., 2020; Navarro et al., 2007) and has been found with a high rate of prevalence in artificial larval habitats along with Aedes (Talaga, 2016).
Toxorhynchites theobaldi and Li. durhamii are ecological regulators of immatures populations and their predator efficiency in PALI is unknown. Therefore, it is not ruled out that both species may have contributed to the occurrence of rare and occasional species (low abundance).
Psorophora ferox (von Humboldt, 1819) and Ps. dimidiata were only collected by CDC and Shannon traps. Psorophora lay their eggs on damp or dry mud where they withstand long periods (months or years) of desiccation and hatch when the habitat is inundated by rain or flood waters (Foster & Walker, 2019). This could potentially explain the higher abundance of both Psorophora species during the rainy period.
There is medical importance associated with a few species in PALI. Several strains of Ilheus and Venezuelan encephalitis viruses have been isolated from Wy. medioalbipes (Wilkerson et al., 2021), but little is known about its medical importance. In Ecuador, the species may be of interest because it was described in Ananas comosus (Navarro et al., 2018), therefore crops can serve as large reservoirs. In fact, Wyeomyia is considered to be underestimated since several pathogens have been recovered from or experimentally passed through species of the genus (Walter Reed Biosystematics Unit, 2023). However, due to problematical identification, there has been no arboviral surveillance. Limatus durhamii is recognized as a vector of VEE virus, Caraparu virus, and Guama virus (Feijó Almeida et al., 2020; Navarro et al., 2015). Psorophora ferox is a vector for Ilheus virus and VEE virus. Also, West Nile virus and Cache, Oriboca, and St Louis encephalitis viruses have been isolated from this species (Wilkerson et al., 2015). Finally, Culex dunni has been reported as a vector of Venezuelan Equine Encephalitis (VEE) (Berti et al., 2013).
Some mosquitoes are known to be able to travel long distances, usually with the help of air currents. In the spread of the Japanese encephalitis virus to Australia, a significant role was played by wind dispersal (Ritchie & Rochester, 2001). Also, in parts of Africa, where surface water is absent for months, malaria persistence is associated with windborne long-distance migration of mosquitoes (Huestis et al., 2019).
Mosquitoes may be able to cross the river, but it will depend on their flight capacity. Mosquitoes generally have a reduced flight range as wind velocity increases (Verdonschot & Besse-Lototskaya, 2014). In general, the flight capacity of Culicidae remains unknown and is likely to vary among species. Information is only available for one of the species recorded in PALI, Ps. ferox, which has excellent dispersal ability (2 500 m of average maximum flight distance), stronger than the most common urban vector species, Aedes aegypti and Ae. albopictus (333 and 676 m, respectively) (Verdonschot & Besse-Lototskaya, 2014).
Psorophora ferox as a vector species could be of concern, as it is more likely to colonize the surroundings of the park than domestic species. But it is known that the migration process is far more complex because it depends not only on flight capacity but also on the prevalence of the species, which is influenced by factors such as the availability of breeding sites and community dynamics. For instance, although urban vector species like Ae. aegypti and Ae. albopictus have been observed in urban parks in Brazil (Ceretti-Junior et al., 2016; Medeiros-Sousa et al., 2015) it is known that under natural conditions, high abundances of Li. durhamii have been observed to lead to competitive exclusion of Ae. aegypti in less disturbed larval habitats (Talaga, 2016).
However, migration from the park to the peri-urban area is not ruled out, particularly for species (Tx. theobaldi, Li. durhamii, Cx. secundus, Cx. derivator, Cx. dolosus) with broad distributions and adaptability to disturbed environments. Furthermore, proximity and environmental degradation may accelerate species migration and lead to epizootic cycles. Vertebrates in PALI (monkeys, bats, rodents, others) contribute to the epidemiological risk. Over time, the park could host more animal populations, potentially maintaining enzootic transmission cycles and even leading to epizootic cycles, that may introduce pathogens into the urban ecosystem. In addition, Hendy et al. (2020) have concluded that the community composition between forest edge and interior sites changes, with a gradual decrease in the abundance of certain species further into the forest. Studies have also concluded that the diversity and abundance of species varies with distance between the ground and the canopy, and that these metrics are concentrated in the lower stratum (Confalonieri & Costa Neto, 2012; Tantely et al., 2019). In other words, arbovirus exchange between humans and wildlife can vary significantly within meters, and that the role of species as possible bridge vectors is likely defined around forest fragments.
Most of the species described have already been reported in Ecuador. However, there is no detailed record of the distribution of Wy. melanopus and Cx. dolosus in the country (Angulo & Olivares, 1993). This may be the first confirmed report of Wy. celaenocephala, and Tx. theobaldi in the Ecuadorian Amazon region, and the first record of Wy. felicia and Cx. derivator for the country, as no records were found after the literature review.
The increase in abundance, including epidemiologically important species (Cx. dunni, Ps. ferox) during the rainy period, warns about the risk of pathogen transmission. Therefore, since rainfall is bimodal, preventive recommendations should be provided to visitors, mainly during periods of high rainfall. Although seasonality was evaluated in this study, precipitation in Tena exceeds 4 000 mm/year, with no months has less than 100 mm (Lucas-Solis et al., 2021), meaning the dry period refers to a period of relatively low precipitation. This may explain why no significant differences were found between seasons, besides abundance.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


