Introduction
Tropical dry forests (TDFs) are strategic ecosystems with great biological diversity and multiple ecosystem services such as soil stabilization, water and climate regulation, carbon storage, among others (Gei & Powers, 2014; Murphy & Lugo, 1986). However, due to the historical loss of more than 60 % of their original coverage, they are among the most threatened ecosystems in the Neotropics (Portillo-Quintero & Sánchez-Azofeifa, 2010). The main causes of loss and TDFs transformation have been the livestock expansion, mining, urban development, and tourism (González-M et al., 2019; Pizano & García, 2014). This situation puts at risk the biodiversity, ecological processes, and ecosystem services in TDFs (Fernandez-Mendez et al., 2014; Quesada et al., 2009). Within the ecological processes that can be affected by land use change, are litterfall, nutrient returns, and decomposition (Gei & Powers, 2014; Meister et al., 2012).
Litterfall, nutrient return and decomposition are fundamental ecological processes for organic matter return and nutrient real return to soils (Vitousek, 1984). These ecological processes represent the ability to transform biomass and supply or retain nutrients depending on the availability of resources (Aerts & Chapin, 1999; Coleman et al., 2018; Vitousek & Sanford, 1986). In this way, in the dry season when resources are scarce, plants in TDFs could reabsorb nutrients before litterfall, as an adaptation mechanism that allows them to be more energy efficient (Gei & Powers, 2014; Vitousek, 1984). Additionally, depending on the degradation level and the ecosystem resilience, these ecological processes can vary widely in different successional stages (Aryal et al., 2015; Sánchez-Silva et al., 2018; Souza et al., 2019; Xuluc-Tolosa et al., 2003).
In advanced successional stages that are distinguished by structural and species composition development (Chazdon, 2014), it has been reported that litterfall and nutrient returns is higher (Barreto da Silva et al., 2018; Castellanos-Barliza et al., 2019; Huang et al., 2017; Sánchez-Silva et al., 2018; Souza et al., 2019). Likewise, the decomposition and nutrients release could be superior and more efficient, due to better soil conditions, greater water efficiency and microbial activity (Schilling et al., 2016). In contrast, other studies have shown that in early and intermediate stages, litterfall, nutrient returns and release may be greater due to fast-growing species with higher photosynthetic rates and efficient nutrient returns (Castellanos-Barliza, León-Peláez, Armenta-Martínez, et al., 2018; Sánchez-Silva et al., 2018; Valdespino et al., 2009; Xuluc-Tolosa et al., 2003). These ecological processes respond to the interaction of multiple biotic factors such as structure and species richness, morphological and physiological traits variation, and microbial activity (Coleman et al., 2018; Steidinger et al., 2019); and abiotic factors such as soil type and microclimatic conditions (Ostertag et al., 2008; Sánchez-Silva et al., 2018; Schilling et al., 2016).
In relation to the N and P foliar nutrients dynamics in TDFs, it has been reported that the P content decreases, and its use efficiency increases with the forest age (Read & Lawrence, 2003); while N returns recovered in the first successional stages (Gei & Powers, 2014). In dry forests of La Guajira in Colombia affected by coal mining, Castellanos-Barliza León-Peláez and Campo (2018) found that after 21 years of active restoration, the flow of N and P recovered, but the rate of P mineralization didn't change. Meanwhile, in dry forests of the Yucatan Peninsula that historically had corn crops, Read and Lawrence (2003) found that N concentration didn't change along plant succession, while the P concentration decreased in mature forests. The P and N flux variation is mainly attributed to its origin nature (Campo et al., 2001; Gei & Powers, 2014); and other factors such as land use history, soil type, climate, and vegetation (Gei & Powers, 2014); but the influence of various abiotic and biotic factors on N and P returns remains uncertain (Souza et al., 2019).
The main objective of this study was to evaluate the litterfall, nutrient potential return and use efficiency, and decomposition variation in a TDF successional gradient. Specifically, we ask the following questions: i) How does the litterfall, nutrient potential return and use efficiency, and decomposition change in different successional stages in a TDF? ii) How is the relationship between some soils chemical properties and vegetation with respect to these ecological process? To answer these questions, we quantified litterfall production for two years (November 2017-October 2019) in four successional stages: initial (3-5 years), early (10-15 years), intermediate, (20-30 years) and late (> 40 years); we identified tree species with the greatest litterfall contribution and quantified the rate decomposition of these species. At the community level we measured C, N and P potential return, N and P use efficiency, C:N and N:P ratios. Finally, we analyzed the relationship of some vegetation characteristics and soil chemical properties with litterfall, the nutrient potential return and use efficiency, and decomposition.
Considering that studies of these ecological processes in TDFs are contrasting due to the biotic and abiotic interactions mentioned above, we expect that: i) Litterfall and decomposition will increase along plant succession, due to the structure development, species richness and better soil conditions, which favor litterfall contribution and nutrients release. ii) Second, we expect N return to be higher in initial and early forests, and P return to increase along succession; in this way N use efficiency and C:N will be higher in late forests, and P use efficiency and N:P will have higher values in the early stages of succession. iii) Third, we believe that soil chemical properties will have a stronger effect on nutrient return and use efficiency, and decomposition; meanwhile the structure and species richness will strongly influence litterfall contribution.
Materials and methods
Study area: The study was conducted at three locations in the upper basin of the Magdalena River, North Tolima department, Colombia. The Civil Society Nature Reserve (CSNR) ''Tambor'' (5°12'25'' N & 74°44'12'' W) in Honda municipality; the CSNR ''Jabiru'' (5°01'50'' N & 74°53'04'' W) in Armero-Guayabal municipality and Hacienda San Felipe (5°07'25'' N & 74°57'06'' W) in Falan municipality (Fig. 1). These forests correspond to TDF remnants in inter Andean valleys Magdalena River. Historically, this zone has been characterized by the establishment of extensive and intensive cattle ranching (Fernandez-Mendez et al., 2014). The geomorphological landscape includes piedmont and low and intermediate hills geoforms. Typic Ustorthents and Lithic Ustorthents are dominant soils, which have developed from sandstone, tuff, and clay (IGAC, 2004). Soil texture is loam to clay loam, with 58.1 % ± 12.9 sand, 25.1 % ± 7.55 silt, and 16.8 % ± 6.12 clay in average. Soils are characterized by medium fertility and moderate pH (García Villalobos, 2020). The climate is hot dry, with a bimodal precipitation, the annual average precipitation is 1 876 ± 258 mm with dry season from January to March and June to September, while the rainy season is from April to May and October to November (Fernandez-Mendez et al., 2014).
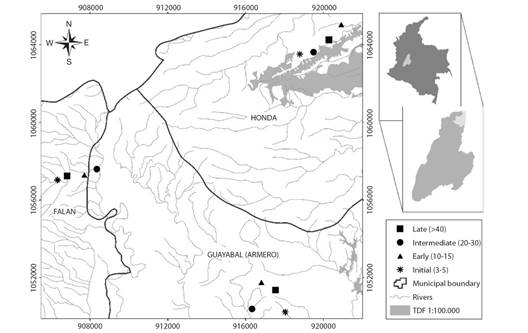
Fig. 1 Study area in North Tolima. Colombia. Permanent monitoring plots (0.18 ha) per successional stage are represented by asterisks for initial (3-5), triangles for early (10-15), circles for intermediate (20-30) and squares for late (> 40).
A total of 12 permanent monitoring plots (PMP) were established (three plots in each successional stage). Since age is not a precise succession metric (Chazdon, 2014), we assigned an approximate age from forest/no forest thematic layers corresponding to 1990, 2000, 2005, 2010 and 2013 years (Salgado-Negret et al., 2017). Additionally, we consider local communities' knowledge regarding age abandonment, in this way age ranges were defined for each successional stage: initial (3-5 years); early (10-15 years); intermediate (20-30); and late (> 40 years). Initial corresponds to sites that have lost their original coverage, which historically were agricultural crops and pastures that were abandoned in the last 5 years. Early characterized by an open canopy, with arboreal and shrubby elements that originated from plant succession in the last 10 to 15 years. Intermediate forests were distinguished by regularly distributed tree elements, forming a discontinuous canopy with trees that reach heights of up to 25 m, tree cover between 30-70 % and an age of abandonment greater than 20 years. Finally, late forests constitute little intervening vegetal formations (sporadic presence of cattle), which are characterized by high natural regeneration, closed canopy and differentiated strata with coverage greater than 70 % (Table 1).
Table 1 Structural characteristics vegetation and floristic composition of study sites of four successional stages in a TDF, North Tolima. Colombia.
| Successional stage | Initial (3-5) | Early (10-15) | Intermediate (20-30) | Late (> 40) |
| Individuals/0.18 ha | 69 ± 33 | 187 ± 105 | 196 ± 67 | 182 ± 56 |
| Individuals /ha | 383 ± 181 | 1 041 ± 585 | 1 087 ± 374 | 1 013 ± 311 |
| Species/0.18 ha | 17 ± 7 | 22 ± 8 | 25 ± 7 | 31 ± 7 |
| Species /ha | 93 ± 39 | 120 ± 42 | 139 ± 39 | 174 ± 37 |
| Basal area m2 0.18 ha-1 | 4.6 ± 1.9 | 21.6 ± 16.9 | 26.7 ± 15.4 | 25.8 ± 14.9 |
| Basal area m2 ha-1 | 25.9 ± 10.7 | 119.7 ± 93.9 | 148.1 ± 85.5 | 143.5 ± 82.6 |
| CWM foliar area (cm2)* | 79.5 ± 40.7 | 64.1 ± 26.7 | 109.3 ± 68.7 | 97.8 ± 33.6 |
| Shannon-Wiener Diversity Index | 2.1 ± 0.3 | 2.2 ± 0.3 | 2.1 ± 0.5 | 2.7 ± 0.4 |
| Species with higher IVI values | Attalea butyracea (Kunth) (28.7 %), Guazuma ulmifolia Lam (24.8 %), Cordia alliodora (Ruiz & Pav.) Oken (22.7 %), Coccoloba coronata Jacq. (20.4 %), y Centrolobium paraense Tul. (17.8 %). | Attalea butyracea (Kunth) (59.1 %), Calliandra tergemina (L.) Benth. (22.3 %), Cordia alliodora (Ruiz & Pav.) Oken (11.1 %) y Eugenia sp. (8.6 %). | Guadua angustifolia Kunth (49 %), Anacardium excelsum (Kunth) Skeels (24 %), Erythroxylum ulei O.E.Schulz (21.7 %), Oxandra espintana (Benth.) Baill. (19.7 %), Attalea butyracea (L.f.) Wess.Boer (19.2 %) y Machaerium tolimense Rudd (10.5 %). | Oxandra espintana (Benth.) Baill. (27.6 %), Anacardium excelsum (Kunth) Skeels (20.1 %), Trichilia oligofoliolata M.E. Morales (|9.2 %), Sorocea cf. sprucei (18.7 %) y Astronium graveolens Jacq. (15.3 %). |
All individuals with DBH ≥ 2.5 cm were included. The values correspond to the mean ± 1 SD of the three study sites. Adapted from Polania, (2019). *Data provided by the IAvH (González-M et al., 2019). CWM: community-weighted mean.
Litterfall production: Litterfall was monitored in 96 circular collectors (0.5 m2) along successional gradient. In each PMP, eight collectors were installed so that 24 collectors per successional state were established following the Biodiversity and Ecosystem Services TDFs Evaluation Protocol (Salgado-Negret et al., 2017). Collectors were permanently exposed and every month for two years litterfall was collected (November 2017-October 2019). To obtain the dry weight, the samples were dried at 60 °C for 24-72 h depending on moisture content (in rainy periods were dried for 48-72 h) until a constant weight was obtained. After drying, the biomass obtained was separated into leaves (refer to foliar litter in this study), branches (< 2 cm) and reproductive material (flowers, fruits). Miscellaneous unidentified material was not considered because it represented less than 5 % of total contribution. Subsequently, the dry weight of each component was obtained in a precision digital scale 0.01 g. Total litterfall was obtained from the monthly production sum of all collectors by successional state, adapting the equation of Honorio and Baker (2010).
To estimate foliar litter contribution by species, every three months in the first year of study (November 2017-October 2018), four collectors per PMP were randomly selected. Subsequently, foliar litter was separated according to their shape and texture, including the leaf blade and the petiole. For foliar litter palms, only the rachis and the pinnae contained in the collector were considered, excluding the petiole and the foliar parts outside the collector (Ribeiro et al., 2020). To estimate contribution by species, foliar litter was weighed on a digital scale with a precision of 0.01, and it was summed by successional stage and by site to selected species that contributed the most foliar litter.
Leaf nutrient potential return and nutrient use efficiency: To evaluate nutrient potential return, four of the eight collectors per PMP were selected, who gathered the quarterly contribution of foliar litter during the first year of study. In total, 192 samples were evaluated (12 plots × 4 collectors × 4 periods in the year). The foliar litter samples were sent to National Laboratory in Bogotá, for carbon (C), nitrogen (N) and phosphorus (P) content quantification. Nutrient potential return was estimated as average annual fluxes (kg ha-1 y-1) multiplying foliar litter production by nutrients concentration in each plot and successional stage (Read & Lawrence, 2003). Additionally, we quantified C:N, N:P ratios and the nitrogen use efficiency index (NUE) and phosphorus use efficiency index (PUE) considering the nutrient efficiency used per unit of dry mass produced in foliar litter, that is equivalent to inverse of foliar litter nutrient concentration (1/(nutrient concentration)) (Vitousek, 1984).
Leaf litter decomposition: To estimate decomposition rates by species and community level, litter bags were built considering the species that contributed the most foliar litter (identified in the previous phase). These species exhibited high IVI values (Table 1) and higher foliar litter contributions, so ecologically they also represented the community level (weighted average of k value). According to Graca et al. (2005); Salgado-Negret et al. (2017), 5 g foliar litter were deposited in 20×20 cm nylon-type bags, with a 5 mm diameter mesh eye. 18 litter bags by individual/species were constructed (522 in total; 22 species and 29 individuals, due to some species were repeated by successional stage). The distribution of litter bags per plot varied between 36 (2 species) to 72 (4 species) according to species selection with the highest foliar litter contribution. The litter bags were installed at the end of May 2019 and were collected at 30, 63, 92, 120, 153 and 189 days. The 18 litter bags per species were installed with a rope that held all the bags to facilitate their collection and avoid their loss; therefore, a separation between the bags was not guaranteed so that they were exposed to the same microclimatic conditions (Salgado-Negret et al., 2017). In the laboratory, the litter bags were carefully cleaned to remove soil particles, emerging fine roots, and other adhering materials. Subsequently, they were dried at 60 °C for 48 to 72 h until reaching a constant weight to estimate the dry weight on a digital scale with 0.01 g precision.
Data processing: To compare litterfall, nutrient potential return - use efficiency, and decomposition across successional stages, we performed one-way Anovas, Welch's robust Anovas, and nonparametric Kruskal-Wallis tests depending on data behavior. Subsequently, to confirm significant differences, we performed Games-Howell post hoc tests or Wilcoxon pairwise multiple comparison with Bonferroni adjustment. Additionally, we performed Principal Component Analysis (PCA) to summarize vegetation characteristics, soil chemical properties, and nutrient potential return and use efficiency for the 12 PMP studied. Thus, we made three PCAs: (i) vegetation characteristics: basal area, leaf area, height and species richness; (ii) soil chemical properties: pH, organic carbon (OC), N, P and cation exchange capacity (CEC) and (iii) potential nutrient return and use efficiency: C, N and P returns, N:P, C:N, NUE and PUE. The scores of the first dimensions (Dim1) of each PCA were selected as a representation of vegetation characteristics, soil chemical properties and nutrients, because they summarized the data and explained most data variance. Finally, to analyze the relationship between the vegetation characteristics and soil chemical properties with litterfall, nutrient potential return and use efficiency, and decomposition, we made three mixed models with the lmer function of the lme4 package. In these models the plot was used as a random factor, and we included three fixed effects: (i). The Dim1 of soil chemical properties PCA; (ii). The Dim1 of vegetation characteristics PCA; and (iii) the interaction of both dimensions (soils×vegetation) (Bates et al., 2015). All analyzes were performed in the R software version 3.6.3 (R Core Team, 2020).
Results
Litterfall production: Total annual litterfall in a TDF range from 4.45 to 8.46 Mg ha-1 y-1 and varied significantly among successional stages. Total litterfall was highest in late and intermediate (Welch, F3,50 = 13.0, P < 0.001) (Fig. 2). This trend was maintained for foliar litter (Welch, F3,50 = 15.8, P < 0.001) and branch fraction (Kruskal-Wallis, H3 = 25.36, P < 0.05), meanwhile the reproductive structures were lower in initial forests and didn't change between early, intermediate, and late forests (Kruskal-Wallis, H3 = 17.04, P < 0.05).
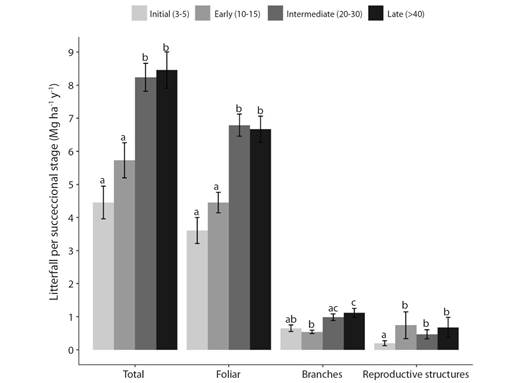
Fig. 2 Total litterfall, foliar, branches and reproductive structures (flowers and fruits) in four successional stages in a TDF, North Tolima. Error bars indicate ± 1 standard error. The values followed by the same letters are not significantly different according to Welch and Kruskal-Wallis test.
Centrolobium paraense, Albizia guachapele and Guazuma ulmifolia were the ones species that contributed the most to leaf litter in initial (41.03 %). Calliandra tergemina, Spondias mombin and Attalea butyracea represented the greatest contribution in early (29.85 %). For its part, Guadua angustifolia, Trichilia oligofoliolata and Anacardium excelsum represented 49.16 % of the leaf litter in intermediate, while A. excelsum, T. oligofoliolata and Oxandra espintiana constituted 33.45 % in late forests (Table 2).
Table 2 Annual leaf litter (Mg ha-1 y-1 ± 1 standard deviation) of species with greatest contribution in four successional stages in a TDF, North Tolima.
| Successional Stage | Species | Leaf litter | % |
| Initial (3-5) | Centrolobium paraense Tul. | 0.53 ± 0.01 | 15.23 |
| Albizia guachapele (Kunth) Dugand | 0.50 ± 0.01 | 14.37 | |
| Guazuma ulmifolia Lam. | 0.40 ± 0.01 | 11.49 | |
| Rondeletia pubescens Kunth | 0.19 ± 0.01 | 5.46 | |
| Cupania latifolia Kunth | 0.19 ± 0.002 | 5.46 | |
| Early (10-15) | Calliandra tergemina (L.) Benth. | 0.53 ± 0.02 | 12.59 |
| Spondias mombin L. | 0.38 ± 0.01 | 9,00 | |
| Attalea butyracea (L.f.) Wess.Boer | 0.35 ± 0.01 | 8.27 | |
| Leguminosae 1 | 0.32 ± 0.01 | 7.76 | |
| Astronium graveolens Jacq. | 0.23 ± 0.01 | 5.58 | |
| Guazuma ulmifolia Lam. | 0.14 ± 0.004 | 3.36 | |
| Leguminosae 2 | 0.14 ± 0.01 | 3.35 | |
| Eugenia sp. | 0.10 ± 0.003 | 2.49 | |
| Intermediate (20-30) | Guadua angustifolia Kunth | 1.68 ± 0.02 | 25.73 |
| Trichilia oligofoliolata M.E. Morales | 0.85 ± 0.03 | 13.02 | |
| Anacardium excelsum (Kunth) Skeels | 0.68 ± 0.03 | 10.41 | |
| Machaerium tolimense Rudd | 0.37 ± 0.03 | 5.67 | |
| Spondias mombin L. | 0.30 ± 0.03 | 4.59 | |
| Bauhinia sp. | 0.27 ± 0.03 | 4.13 | |
| Oxandra espintana (Benth.) Baill. | 0.24 ± 0.03 | 3.68 | |
| Machaerium microphyllum (E.Mey.) Standl. | 0.20 ± 0.03 | 3.06 | |
| Late (> 40) | Anacardium excelsum (Kunth) Skeels | 1.14 ± 0.01 | 17.43 |
| Trichilia oligofoliolata M.E. Morales | 0.65 ± 0.02 | 9.94 | |
| Oxandra espintana (Benth.) Baill. | 0.39 ± 0.02 | 5.96 | |
| Ocotea longifolia Kunth | 0.36 ± 0.02 | 5.5 | |
| Rutaceae | 0.36 ± 0.02 | 5.5 | |
| Attalea butyracea (L.f.) Wess.Boer | 0.30 ± 0.02 | 4.59 | |
| Ampelocera albertiae Todzia | 0.26 ± 0.02 | 3.98 | |
| Machaerium tolimense Rudd | 0.20 ± 0.01 | 3.06 |
Leaf nutrient potential return and nutrient use efficiency: The C, N and P potential return, N and P use efficiency, and C:N didn't change throughout succession (P > 0.05). N:P was the only variable that exhibited differences between initial and intermediate/late forests (Kruskal-Wallis, H3 = 8.34, P < 0.05) (Table 3). Even so, nutrient potential return and use efficiency presented an important variation along succession conditioned by the site. C, N and P return tended to be greater in intermediate and late stages. This can be explained because although Jabirú exhibited high nutrient return in all successional stages, Tambor and San Felipe presented lower values in initial and early stages to induce a lower average value in the first stages. For its part, PUE reached mean higher values in initial and early successional stages; meanwhile, NUE and C:N didn´t present clear differences in succession (Table 3).
Table 3 Carbon (C), nitrogen (N) and phosphorus (P) potential return (kg ha-1 y-1), nitrogen use efficiency (NUE) and phosphorus use efficiency (PUE), C:N and N:P (mean ± 1 standard deviation) in four successional stages in a TDF, North Tolima.
| Succesional stage | Initial (3-5) | Early (10-15) | Intermediate (20-30) | Late (> 40) |
| C (kg ha-1 y-1) | 1 961.53 ± 830.92 | 1 841.53 ± 159.74 | 2 773.1 ± 317.53 | 2 758.37 ± 27.23 |
| N (kg ha-1 y-1) | 85.77 ± 43.47 | 63.53 ± 11.46 | 94.83 ± 12.18 | 90.03 ± 12.56 |
| P (kg ha-1 y-1) | 5.33 ± 3.61 | 4.73 ± 1.93 | 8.07 ± 1.13 | 6.13 ± 1.28 |
| NUE | 70.17 ± 16.11 | 70.13 ± 6.68 | 72.53 ± 11.24 | 78.27 ± 14.65 |
| PUE | 1 624.9 ± 556.07 | 1 576.23 ± 815.64 | 958.23 ± 279.99 | 1 166.43 ± 232.57 |
| C:N | 32.93 ± 7.86 | 30.97 ± 2.51 | 30.67 ± 3.52 | 33.3 ± 4.6 |
| N:P | 23.1 ± 4.8a | 22.17 ± 9.72 | 13.03 ± 2.18b | 14.4 ± 2.14b |
Letters indicate significant differences between successional stages (P < 0.05).
In this sense, nutrient potential return and use efficiency exhibited a clear change between study sites. Nutrients PCA confirmed it because this allowed a clear site separation. Jabirú were related to high nutrient potential return; while San Felipe exhibited high N:P and PUE; and Tambor associated with C:N and NUE. DIM1 explained 68.2 % variance, allowing to describe the nutrient potential return and use efficiency.
Leaf litter decomposition: Decomposition rates are higher in initial and early compared to intermediate and late forests (F2,9 = 1.62, P = 0.05). The residual dry mass-RDM varied from 85.7 % (month 1) to 54.2 % (month 6) in initial forests; 88.2 to 45.4 % in early forests; 90.9 to 61.7 % in intermediate forests; and 92.1 to 63.4 % in late forests. At the species level, R. pubescens and G. ulmifolia presented the highest decomposition rates and the shortest RDM in initial; Leguminosae 1 and A. graveolens in early; S. mombin and O. espintiana in intermediate; and Rutaceae and O. espintiana in late (Table 4, Fig. 3). The R2 values ranged from 0.83 to 1, indicating that the model appropriately described the decomposition rates for all species in all successional stages.
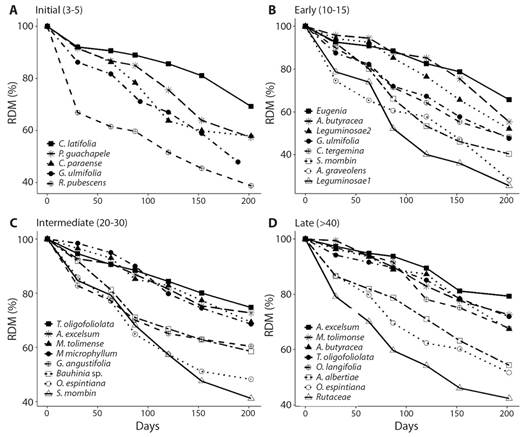
Fig. 3 Residual dry mass of foliar litter for the species with the highest litterfall contribution in four successional stages in a TDF, North Tolima.
Table 4 Decomposition factor k, decomposition time of 50 % and 99 % of foliar litter for species in in four successional stages in a TDF, North Tolima.
| Successional Stage | Species | k | t 0.5 (years) | t 0.99 (years) | R2 adj |
| Initial (3-5) | R. pubescens | 1.51 | 0.58 | 3.84 | 0.9 |
| G. ulmifolia | 1.33 | 0.52 | 3.46 | 0.98 | |
| C. paraense | 1.10 | 0.46 | 3.05 | 0.93 | |
| A. guachapele | 1.02 | 0.68 | 4.51 | 0.96 | |
| C. latifolia | 0.58 | 1.20 | 7.94 | 0.91 | |
| Early (10-15) | Leguminosae 1 | 2.5 | 0.28 | 1.84 | 0.97 |
| A. graveolens | 1.97 | 0.35 | 2.34 | 0.92 | |
| S. mombin | 1.79 | 0.39 | 2.57 | 0.97 | |
| C. tergemina | 1.35 | 0.51 | 3.41 | 0.99 | |
| G. ulmifolia | 1.31 | 0.53 | 3.52 | 0.99 | |
| Leguminosae 2 | 1.17 | 0.59 | 3.94 | 0.93 | |
| A. butyracea | 0.98 | 0.71 | 4.70 | 0.83 | |
| Eugenia | 0.69 | 1.00 | 6.67 | 0.93 | |
| Intermediate (20-30) | S. mombin | 1.66 | 0.65 | 4.34 | 0.99 |
| O. espintiana | 1.39 | 0.50 | 3.31 | 0.95 | |
| Bauhinia sp | 1.01 | 0.69 | 4.56 | 0.94 | |
| G. angustifolia | 0.87 | 0.80 | 5.29 | 0.87 | |
| M. microphyllum | 0.75 | 0.92 | 6.14 | 0.95 | |
| M. tolimense | 0.67 | 1.03 | 6.87 | 0.98 | |
| T. oligofoliolata | 0.59 | 1.17 | 7.81 | 0.97 | |
| A. excelsum | 0.51 | 1.17 | 7.81 | 1 | |
| Late (> 40) | Rutaceae | 1.55 | 0.45 | 2.97 | 0.96 |
| O. espintiana | 1.17 | 0.59 | 3.94 | 0.97 | |
| A. albertiae | 1.05 | 0.66 | 4.39 | 0.98 | |
| O. longifolia | 0.75 | 0.92 | 6.14 | 0.94 | |
| A. butyracea | 0.68 | 1.02 | 6.77 | 0.92 | |
| M. tolimense | 0.63 | 1.10 | 7.31 | 0.97 | |
| T. oligofoliolata | 0.58 | 1.20 | 7.94 | 0.97 | |
| A. excelsum | 0.45 | 1.54 | 10.23 | 0.93 |
The adjusted R2 indicates the coupling of the exponential model used for each species (P < 0.05).
Relationship with vegetation characteristics and soil chemical properties: Litterfall exhibited a positive relationship with vegetation characteristics (x2 = 28.96; P < 0.05) (Fig. 4A), but no clear relationship was found with soil chemical properties (x2 = 0.89; P > 0.05) (Fig. 4B). The interaction between soil chemical properties and vegetation characteristics had a slight relationship with litterfall (x2 = 3.14; P = 0.076). In general, intermediate, and late forests exhibited higher contributions of litterfall, better structural development of vegetation and higher species richness, but not necessarily better soil conditions.
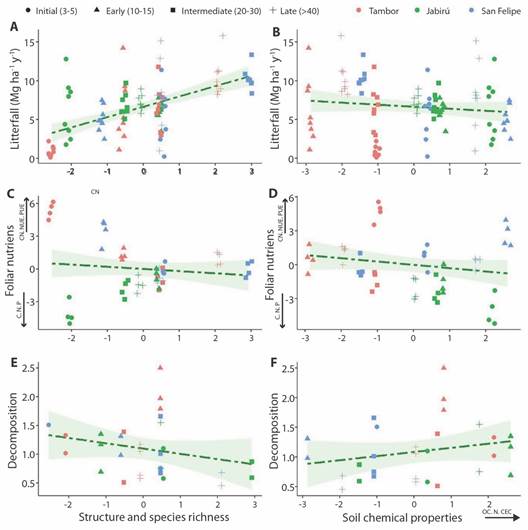
Fig. 4 A. Annual litterfall (Mg ha-1 y-1) in relation to vegetation characteristics (vegetation PCA Dim1 score) and B. soil chemical properties (soil PCA Dim1 score) (N = 96, litter collectors). C. Nutrient potential return and use efficiency in relation to vegetation characteristics and D. soil chemical properties (N = 48, collectors for nutrient and soil analysis). E. Decomposition rate (k) in relation to vegetation characteristics and F. soil chemical properties (N = 29, species).
In contrast, nutrient potential return and use efficiency were not related to vegetation characteristics (x 2 = 2.82; P > 0.05) (Fig. 4C); but showed a positive relationship with soil chemical properties (x 2 = 4.28; P = 0.04) (Fig. 4D). High C:N and N, P use efficiency were related to low nutrients, OC % and CEC in soils; while nutrient potential return (N, P) were related to high chemical soil properties values. Jabirú presented the highest nutrient potential return and better soil chemical conditions, while San Felipe and Tambor exhibited the highest use efficiency.
Finally, the decomposition rate presented a relationship with the soil-vegetation interaction (x2 = 4.27; P = 0.04). However, when evaluating only the structure and species richness, there wasn't relationship with decomposition (x2 = 3.21; P > 0.05) (Fig. 4E); Likewise, the soil chemical properties were not related to decomposition (x2 = 0.14; P > 0.05) (Fig. 4F).
Discussion
The plant succession favored the recovery of litterfall and decomposition; while the site conditions associated with soil chemical properties were determinant in nutrient dynamics. Soil chemical properties, vegetation structure and species richness of intermediate and late forests favored a greater litterfall contribution. Regardless of the successional stage, better soil conditions favored foliar nutrient returns and their release through decomposition. These results suggest that tropical dry forests in different successional stages have the capacity to recover key ecological processes related to nutrient cycling and NPP, although the soil quality conditioned by land use history has a great influence on nutrients dynamics.
Litterfall recorded in this study (4.45-8.46 Mg ha-1 y-1) was within the range reported in similar forest ecosystems (Aryal et al., 2015; González-Rodríguez et al., 2011). In this regard, litterfall was low in initial and early stages and increased in intermediate and late forests, due to the recovery of the structure and species richness. In general, these results support our first hypothesis and coincided with several studies in TDFs in Mexico (Morffi-Mestre et al., 2020; Sánchez-Silva et al., 2018), Costa Rica (Schilling et al., 2016) and Brazil (Souza et al., 2019), who's attributed litterfall increase to the biomass superiority and canopy development.
The high litterfall contribution in intermediate and late forests can also be explained by the species composition and their morphological and physiological traits (Becknell & Powers, 2014; Lopezaraiza-Mikel et al., 2014; Villalobos et al., 2014). In this study we found a positive effect of species richness and leaf area on litterfall, which could be attributed to litterfall would be mediated by significant contribution of dominant individual trees (abundance and basal area) with large leaves in more diverse sites (Clark et al., 2001; Huang et al., 2017). In this way, the role of the dominant species was fundamental in litterfall contribution, particularly in foliar litter, supported by the biomass ratio hypothesis (Grime, 1998), which suggests that ecosystem processes are determined by dominant species (Conti & Díaz, 2013; Finegan et al., 2015).
Nutrients from litterfall are key to maintaining soil stability and supplying resources to microorganisms and plants (Gei & Powers, 2014; Hulshof et al., 2014; Schilling et al., 2016). In this study, the average C foliar return in late forests was like that reported in primary dry forests of Calakmul, Mexico (Aryal et al., 2015). The average N and P foliar return was higher than that recorded in various TDFs of Mesoamerica such as the Calakmul primary forests (Aryal et al., 2015), Chamela, Mexico (Campo et al., 2001) and secondary forests (7 years old) in Santa Martha, Colombia (Castellanos-Barliza, León-Peláez, Armenta-Martínez et al., 2018). These differences can be attributed to multiple factors at the regional scale like the climate variation, land use history and age forest, topographic and soil conditions (Campo, 2016; Gei & Powers, 2014; Waring et al., 2021); and local variations in species composition and soil microorganisms (Coleman et al., 2018; Schilling et al., 2016).
Nutrient fluxes can vary widely due to climatic seasonality, forest age, soil condition, and species composition (Campo, 2016; Campo & Merino, 2019; Gei & Powers, 2014; Saynes et al., 2005). Similarly, to results of (Castellanos-Barliza León-Peláez, Armenta-Martínez et al., 2018; Valdespino et al., 2009) in Colombian and Mexican TDFs respectively, we found that nutrient potential return was mainly conditioned by nutrients availability in soils, which supports our third hypothesis and allowed a differentiation between study sites. Meanwhile, contrary to what we expected the variation of nutrient potential return and use efficiency didn't change along plant succession. The N:P was the only variable that changed in plant succession, being higher in initial compared to intermediate and late forests; however, this result was conditioned by the site effect in the early forests. In this respect, plants in TDFs can supply their P requirement by the accumulation of soil P bicarbonate and dissolved P pools during the dry season (Valdespino et al., 2009), and the presence of ectomycorrhizal fungi (Meeds et al., 2021).
After litterfall, decomposition process begins, which allows the gradual nutrients release to the soil (Cornwell et al., 2008). We found that rate decomposition was higher in initial and early forests, and better soil chemical conditions, structure vegetation and species richness favored decomposition. These results partially support our hypothesis, since the soil-vegetation interaction favored nutrients release, but contrary to what we expected (first hypothesis), decomposition was higher in initial forests. This can be explained by species composition with rapid nutrient return (e.g., G. ulmifolia, R. pubescens and S. mombin). This coincides with what was reported in Southwest Mexico TDFs (Sánchez-Silva et al., 2018) and the Yucatán Peninsula (Xuluc-Tolosa et al., 2003), who found that early-stage species exhibit high rates of decomposition due to high N foliar concentration.
Decomposition rates can vary along plant succession due to the interaction between fauna, substrate, quality foliar litter and microclimatic conditions (Cornwell et al., 2008; Schilling et al., 2016). Thus, higher decomposition rates in the initial and early forests can also be explained by the microclimatic conditions of sunlight and rain direct entry, which could favor nutrients release of species adapted to water deficit (Sánchez-Silva et al., 2018; Xuluc-Tolosa et al., 2003). However, in this study we don't directly analyze microclimatic conditions, but we found an effect of successional state. In this way, we suggest that soil condition and species composition of each successional stage were determining factors in the differences found in decomposition rates (Coleman et al., 2018; Schilling et al., 2016).
The results of this study show that changes in the litterfall contribution, nutrient potential return and use efficiency, and decomposition can be indicators of biogeochemical cycles recovery in TDFs (Castellanos-Barliza, León-Peláez, & Campo et al., 2018; Celentano et al., 2011; Gei & Powers, 2014). These ecological processes could be used in ecological restoration monitoring programs, with emphasis on the ecosystem functions recovery and soil restoration (Celentano et al., 2011; Restrepo et al., 2013). Another relevant aspect in ecological restoration is the species selection according to land degradation, landscape context, economic and social factors (Lamb & Gilmour, 2003). In this way, these ecological processes provide valuable information for species selection that facilitate biogeochemical cycles reestablishment (Celentano et al., 2011).
Applied to ecological restoration, fast-growing species with a high nutrient return and high decomposition rates (e.g., G. ulmifolia, R. pubescens and C. paraense) could be incorporated in degraded sites, which help to recover the soil structure (Castellanos-Barliza et al., 2019) In early stages, species such as A. graveolens and S. mombin can favor the continuous supply of litterfall and rapid nutrient release (associated with high decomposition), promoting better microclimatic conditions for the gradual species of advanced stages of succession establishment. On the other hand, in intermediate and late forests, T. oligofoliolata and A. excelsum are key species in net primary productivity and carbon storage, due to their high production and accumulation of litterfall.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


