Introduction
Seasonal tropical forests exhibit a significant variation in plant phenological, causing temporal patterns in fruit availability throughout the year (Bullock & Solis-Magallanes, 1990; Morales-Pérez, 2005; Quigley & Platt, 2003). Under these conditions, animals may employ different foraging strategies in response to changes in food resource availability (Fleming, 1992; Leighton & Leighton, 1983). Psittaciformes (cockatoos, macaws, parrots, and the like) feed on fruits, seeds, and flowers of different plant species (Galetti, 1993; Renton, 2001; Renton et al., 2015), which show high phenological variability in their productivity (Hilty, 1980; Loiselle & Blake, 1991). Given the variation in the food supply, parrots make seasonal movements between habitats, and they show fluctuations in local abundance and dietary switching (Renton et al., 2015). In addition, parrots can adjust the dietary niche breadth or select resources according to food resources abundance (De Labra-Hernández & Renton, 2019; Díaz et al., 2012; Renton, 2001; Robinet et al., 2003). However, the continuous degradation of the tropical forests that the parrots inhabit causes landscapes with food resources distributed in discontinuous patches of remnant forest (Laurance, 1999; Saunders, 1990). Therefore, generating information on the availability and use of food resources in modified landscape is essential to identify foraging areas and the most important plant species in the diet of parrots populations (De la Parra-Martínez et al., 2019; Rivera et al., 2020). Thus, these data can be used as a conservation tool for this critically endangered group of birds (Berkunsky et al., 2017; Olah et al., 2016).
The Orange-fronted Parakeet (OFP) (Eupsittula canicularis) has a wide distribution from the Mexican Pacific slope (Southeast of Sinaloa) to Northwestern Costa Rica (Forshaw, 1989; Howell & Webb, 1995). It is one of the most common psittacines in the deciduous and semi-deciduous tropical forest and is considered endemic to Mesoamerica (Palomera-García, 2010). However, the rapid destruction of its habitat (e.g. 48.6 % loss of OFP historical distribution area in Mexico) due to the conversion of primary forests to secondary vegetation and illegal poaching have caused a population decline (BirdLife International, 2018; Cantú-Guzmán et al., 2007; Monterrubio-Rico et al., 2016; Pires, 2012). Despite this, there are information gaps on the OFP foraging ecology. In Mexico, there are lists of different plant species that are part of the OFP diet (De la Parra-Martínez et al., 2016; Eguiarte & del Río, 1985; Palomera-García, 2010). Additionally, it has been reported that, in degraded areas, the species can feed resources from corn, sorghum, and fruit crops (González-Gómez, 2018; Palomera-García, 2010). However, OFP foraging strategies in modified landscapes have not yet been assessed.
In Mexico, anthropogenic activities have severely degraded the tropical deciduous forest (De Jong et al., 2010; Trejo & Dirzo, 2000). In the state of Oaxaca, it is estimated that tropical deciduous forest covers an area of 26 731 km2 (28.8 % of the state), but only 52.4 % remains preserved (Meave et al., 2012). On the coast of Oaxaca, particularly in the municipality of Santa María Colotepec, where this study was carried out, 67.8 km2 of primary forest were deforested between 2000 and 2011, while agricultural and livestock areas increased by up to 75 %; 36.9 km2 and 44.7 km2, respectively (Leija-Loredo et al., 2016). The loss of primary forests could modify the food resource available for the OFP. Therefore, this study aims to determine spatial variability in food resource abundance, diet, and foraging behavior (particularly dietary niche breadth and resource selection) of OFP in a modified landscapes in Oaxaca Coast, México.
Materials and methods
Study site: The study was carried out in the Coastal region of Oaxaca in Mexico, specifically in the town of Camalote (15°56'03'' N & 96°52'27'' W) and Corozalito (15°54'09'' N & 96°49'58'' W) belonging to the municipality of Santa María Colotepec, covering an area of 30.7 km2. These localities have both conserved areas of primary forest (tropical deciduous and semi-deciduous forest) and degraded areas made up of pastures, crops, and secondary forest. The climate is warm and subhumid, with rains in summer (Aw0), and the temperature varies between 24-28 °C. The dry season occurs from November to May, while the rainy season occurs from June to October; precipitation varies between 800-2 000 mm (Trejo, 2004).
The predominant vegetation is deciduous forest (DF), where tree species measuring between 7-15 m in height, such as Ceiba parvifolia, Guaiacum coulteri, Bursera sp., Guazuma ulmifolia, and Jacaratia mexicana (Rzedowski, 2006). In addition, semi-deciduous forest (SDF) is present in the most humid areas, such as ravines and along rivers, with 20-30 m tall trees such as Tabebuia rosea, Bursera simaruba, Ficus insipida, Astronium graveolens and Homalium trichostemon (Rzedowski, 2006). In recent decades, 67.8 km2 of primary forest were deforested (Leija-Loredo et al., 2016), and most of this area is now secondary forest in different stage of succession.
Food resources availability: On each forest types (DF, SDF, and SF), 30 phenology transects (200 × 6 m) were established to determined food availability for the OFP (Chapman et al., 1994). From February to June 2019, 250 fruiting trees with a diameter at breast height (DBH) ≥ 10 cm were marked and monitored to record DBH and estimated fruit abundance index was measured by multiplying tree DBH by the proportion of fruits in the canopy of each tree (Boyes & Perrin, 2010; Chapman et al., 1994). In order to make comparisons between forest types, the following variables were considered in each phenology transect: (1) number of fruiting trees, (2) number of fruiting tree species, and (3) fruiting abundance index. Tree species were identified in the field or using botanical sampling collected for later identification at the Laboratory of Biological Collections of the Universidad del Mar, campus Puerto Escondido, Oaxaca, with the help of specialized bibliography (García-Mendoza & Meave, 2011; Pennington & Sarukhán, 2005; Pérez & Barajas-Morales, 2011; Salas-Morales et al., 2003; Salas-Morales et al., 2007).
Orange-fronted Parakeet diet: The OFP diet was determined by focal observations of feeding activity during the first four hours after sunrise (06:30-10:30 h, N = 112 hours of observation), along different survey routes in the same areas where phenology transects were established. Each survey route was visited once a week from February to June 2019. Foraging records were obtained by walking in one direction within the routes and stopping only to record OFP feedig activity. In addition, opportunistic observations were considered when observing the OFP feeding off the routes and during evening observations. For each foraging activity, date, time, habitat type, number of parakeets, tree species, and tree part consumed (fruit, seed, leaf, flower or wood) were recorded. A single foraging record was considered when observing one or more parakeets feeding in a tree; when the parakeets change to another resource item or tree, it was considered a second feeding record (Galetti, 1993).
Data analysis: Data from the phenology transects were used to determine food resource abundance and availability for the OFP. Shapiro-Wilk normality tests were applied of data from the phenology transects. Number of fruiting trees variable presented a normal distribution, while the fruiting abundance index presented a normal distribution only after log transformation. For this reason, analysis of variance (ANOVA) was applied to determine spatial (DF, SDF, and SF) differences in food resource availability with the post hoc Tukey-Kramer test (Zar, 1999). The number of fruiting tree species was not normally distributed even after log transformation. Therefore, a non-parametric Kruskal-Wallis test was applied to determine food resource availability between forest types (Zar, 1999). Analyses were performed in R version 3.3.0 (R Core Team, 2016).
To assess the OFP dietary niche breadth, standardized Levin's index was estimated as Best = (B-1) / (n-1) where: B is Levin's index that includes the proportion of parakeets that fed on each resource, and n is the number of resources used (Krebs, 1999; Levins, 1968). Index expresses the breadth of the niche on a scale that varies from 0 (narrow niche), indicating that the use is concentrated on few resources, to 1 (broad niche), indicating that the use is equally distributed over the resources used (Colwell & Futuima, 1971). On the other hand, Hurlbert niche index (Hurlbert, 1978) was calculating to evaluated the OFP dietary selection, which estimates the niche breadth considering the proportion used and the availability of each resource. Index is expressed as Ha = S(pij/âi) where: pij is the proportion of the resources used by the parakeets and âi is the quantity or availability of each resource (Krebs, 1999). In this case, a value close to 0 indicates dietary selection, while a value close to 1 indicates that dietary resources are consumed based on availability, without selection. Additionally, G-test with 95 % Bonferroni confidence intervals (CI) was performed to determine whether use of resources by the OFP differed significantly from that expected by their availability in each forests type, and to determine whether the frequency of foraging by OFP recorded in each forests type corresponded to that expected according to the fruiting abundance index (Byers et al., 1984; Neu et al., 1974).
Results
Food resources availability: A total of 34 tree species grouped into 20 families were recorded in the phenology transects. Species richness was higher in DF (N = 21 species), followed by SDF (N = 18 species), and SF (N = 9 species). Considering the three forest types, the Fabaceae family predominated with nine trees species. In the DF, the most abundant tree species were Jacaritia mexicana (N = 25 trees; Caricaceae), Cocholspermum vitifolium (N = 22 trees; Cochlospermaceae), and Guazuma ulmifolia (N = 16 trees; Malvaceae). In the SDF, the most abundant tree species were Homalium trichostemon (N = 29 trees; Salicaceae), Inga vera (N = 19 trees; Fabaceae), and Bursera simaruba (N = 17 trees; Burseraceae). While in the SF, the most abundant tree species were G. ulmifolia (N = 17 trees) and Heliocarpus pallidus (N = 13 trees; Malvaceae). ANOVA analyses demonstrated significant differences for the number of fruiting trees by forest types (F2,27 = 10.6, P < 0.001); the DF had significantly higher fruiting trees during February and March (Fig. 1A). Nevertheless, the number of fruiting tree species by forest type did not differ significantly (H2 = 0.01, P > 0.05, Fig. 1B). Regarding the fruiting abundance index, there were statistically significant differences between forest types (F2,27 = 5.7, P = 0.008); the SDF had significantly higher food resources availability mainly during April to June (Fig. 1C).
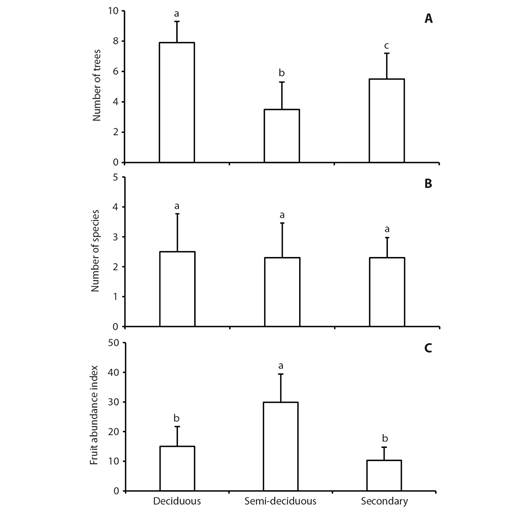
Fig. 1 Mean (± SD) of the availability of food resources in three forests types in Santa María Colotepec, Coast of Oaxaca, Mexico during the middle to end dry season. A. number of fruiting trees, B. number of fruiting species, C. fruiting abundance index (S DBH x the proportion of fruits in the canopy). Different letters on the bars indicate significant differences (Tukey-Kramer, P < 0.05).
Orange-fronted Parakeet diet: During the study period, 41 OFP feeding records (N = 201 individuals) was obtained on 13 plant species from 10 families (Table 1). Of the all OFP feeding records, 42.3 % (N = 85 parakeets) corresponded to fruits, 29.3 % (N = 59 parakeets) to seeds, and 28.4 % (N = 57 parakeets) flowers.
Table 1 Tree species that are part of the diet of Eupsittula canicularis during the middle to end dry season in Santa María Colotepec, Oaxaca. In addition, number of foraging records, number of individuals feeding, tree part consumed, and date of the record are included.
| Family / Species | Common name | Tree part consumed | Foraging records | Number of parrots | Month |
| Anacardiaceae / Mangifera indica | Mango | Fruit (pulp) | 4 | 17 | April May |
| Burseraceae / Bursera simaruba | Palo mulato | Seed | 5 | 16 | March/May |
| Burseraceae / Bursera sp. | Copal | Seed | 1 | 6 | April |
| Caricaceae / Jacaratia mexicana | Bonete | Seed | 2 | 4 | April |
| Fabaceae / Gliricidia sepium | Cacahuanano | Flower | 3 | 37 | February/April |
| Fabaceae / Senna sp. | candelillo | Seed | 1 | 2 | February |
| Malpighiaceae / Byrsonima crassifolia | Nanche | Seed | 3 | 12 | April -May |
| Malvaceae / Guazuma ulmifolia | Caulote | Seed | 1 | 8 | February |
| Moraceae / Ficus cotinifolia | frutillo | Fruit (syconium) | 5 | 39 | February-April |
| Moraceae / Ficus insipida | Higo | Fruit (syconium) | 5 | 22 | May-June |
| Rubiaceae / Chomelia spinosa | Malacahuite | Seed | 2 | 9 | April |
| Salicaceae / Homalium trichostemon | Palo de piedra | Flower | 4 | 22 | February/March |
| Urticaceae / Cecropia obtusifolia | Chancarro | Fruit | 5 | 7 | February, April, May |
Regarding the forest types, the principal items consumed in DF were fruits of F. cotinifolia (39.5 %), and seeds of Bursera sp (29.6 %). In the SDF, the most frequent food item were fruits of F. insipida (32.8 %), followed by flowers of H. trichostemon (28.6 %), and seeds of B. simaruba (21.4 %). In the SF, the main foot item were fruits (pulp) of Mangifera indica (29.3 %), followed by the seeds (20.7 %) of G. ulmifolia, along with flowers (11.7 %) of G. sepium.
Dietary niche breadth and resource selection: The niche breadth was broad (Best = 0.619), with seven plants contributing > 5 % of the OFP diet. Nevertheless, F. cotinifolia (19.4 %), and G. sepium (18.4 %), were consumed more by the OFP. However, differences in the dietary niche breadth were found when separating the analysis by forest types. In the DF, the niche breadth was moderately (Best = 0.505) which indicates that the OFP generally uses few food resources, where F. cotinifolia, G. sepium, and Bursera sp. contributed > 5 % of the diet. In the SDF, the niche breadth was narrower (Best = 0.450), the use of food resources by the OFP is concentrated on few resources; where F. insipida and H. trichostemon had the highest number of parakeets feeding (N = 44). In the SF, the niche breadth remained broad (Best = 0.637), with four species (M. indica, G. sepium, B. crassifolia, and G. ulmifolia) representing > 5 % of the OFP diet.
Hurlbert niche index was narrower (H a = 0.295), which suggests that the OFP selects food resources (G11 = 58.5, P < 0.001). Bonferroni 95 % CI (Table 2), showed that in DF, the OFP consumed F. cotinifolia, G. sepium, C. spinosa, Bursera sp., F. insipida, and Bursera sp., more than expected from their availability (Fig. 2A). Also, J. mexicana is consumed in a significantly lower proportion than expected (Fig. 2A). In SDF, F. insipida and F. cotinifolia are consumed in more significant proportion than expected from their availability (P < 0.05, Fig. 2B). Also, H. trichostemon and B. simaruba are consumed in a significantly lower proportion than expected (Fig. 2B). In SF, M. indica and B. crassifolia are consumed in more significant proportion than expected from their availability (Fig. 2C). Also, G. ulmifolia is consumed in a significantly lower proportion than expected (Fig. 2C). Conversely, the consumption of all other plant species did not differ significantly from that expected according their availability (Fig. 2C).
The frequency of OFP foraging records differed significantly in each forest type (G2 = 34.3, P < 0.01, Fig. 3). In particular, the Bonferroni 95 % CI indicate that OFP feeds more in the DF (Pobs = 0.40, 95 % CI = 0.293-0.583) in accordance with the greater fruiting abundance index (Pesp = 0.27). In the SDF, OFP feeds a significantly lower proportion (Pobs = 0.33, 95 % CI = 0.195-0.471) than expected from their availability of food resources (Pesp = 0.54, P < 0.05). Finally, in the SF, OFP feeds (Pobs = 0.29) did not differ significantly from that expected (P esp = 0.18; P > 0.05); the OFP feeds as would be expected from the fruiting abundance index
Table 2 Food resource availability and use by Eupsittula canicularis, with Bonferroni confidence intervals (95 %), during the middle to end dry season in three forest types in Santa María Colotepec, Coast of Oaxaca, Mexico.
| Vegetation | Tree species | Available proportion | Usage Proportion | Bonferroni confidence intervals |
| DF | Ficus cotinifolia | 0.07 | 0.40 | 0.33 ≤ obs ≤ 0.51* |
| Gliricidia sepium | 0.00 | 0.30 | 0.23 ≤ obs ≤ 0.40* | |
| Bursera sp. | 0.00 | 0.07 | 0.03 ≤ obs ≤ 0.13* | |
| Jacarantia mexicana | 0.93 | 0.05 | 0.01 ≤ obs ≤ 0.09* | |
| Chomelia spinosa | 0.00 | 0.11 | 0.06 ≤ obs ≤ 0.18* | |
| Cecropia obtusifolia | 0.00 | 0.07 | 0.00 ≤ obs ≤ 0.03 | |
| SDF | Ficus insipida | 0.04 | 0.33 | 0.25 ≤ obs ≤ 0.41* |
| Homalium trichostemon | 0.59 | 0.33 | 0.25 ≤ obs ≤ 0.41* | |
| Bursera simaruba | 0.35 | 0.24 | 0.16 ≤ obs ≤ 0.31* | |
| Ficus cotinifolia | 0.02 | 0.10 | 0.05 ≤ obs ≤ 0.16* | |
| SF | Mangifera indica | 0.00 | 0.32 | 0.21 ≤ obs ≤ 0.38* |
| Gliricidia sepium | 0.00 | 0.13 | 0.15 ≤ obs ≤ 0.30 | |
| Byrsonima crassifolia | 0.00 | 0.23 | 0.13 ≤ obs ≤ 0.30* | |
| Guazuma ulmifolia | 0.94 | 0.15 | 0.07 ≤ obs ≤ 0.28* | |
| Senna sp. | 0.06 | 0.13 | 0.05 ≤ obs ≤ 0.16 | |
| Cecropia obtusifolia | 0.00 | 0.04 | 0.00 ≤ obs ≤ 0.07 |
DF = tropical deciduous forest, SDF = tropical semi-deciduous forest, and SF = secondary forest. *P < 0.05.
Discussion
Food resource availability: Food resource availability for the OFP demonstrated spatial fluctuations at the study site. In particular, the SDF produced greater food resource abundance in April to June (end dry season). The findings are consistent with that found by Renton (2001) in tropical deciduous forest of Jalisco, México, where there was a greater food abundance for the Lilac-crowned Amazon (Amazona finschi) in the SDF during the early to end dry season. Food resource availability plays an important role on parrot reproduction (Renton, 2002; Renton & Salinas-Melgoza, 2004; Rivera et al., 2020). The OFP breeds in the dry season during January to June (Collar et al., 2022; De Labra-Hernández, 2022; Palomera-García, 2010). The pattern of resource availability demonstrates the importance of SDF in providing essential food resources during the OFP breeding season (raising young period) (Collar et al., 2022). Also, the DF may be important in providing food resources at the February to March, when OFP are egg laying and during hatching period (De Labra-Hernández, 2022). Conversely, the SF produced little food resource abundance. However, the SF could supplement the food demand that the OFP requires at the end breeding season in April to May. In fragmented forests, SF provided complementary resources for parrots species during the breeding season, as reported for the Northern Mealy Amazon (Amazona guatemale) (De Labra-Hernández & Renton, 2019). The information generated in this study highlights the need to maintain the complete forest structure in a modified landscape to ensure food resources availability for OFP during the breeding season.
Orange-fronted Parakeet diet: Fruits (42.3 %) were the most common item eaten by the OFP, supporting the results by Palomera-García (2010) in tropical deciduous forest of Colima, México. In general, smaller-bodied parrot species consume more fruit (22-44 %) in the diet, as reported in other psittacine species (Galetti, 1997; Hingston et al., 2004; Renton et al., 2015).
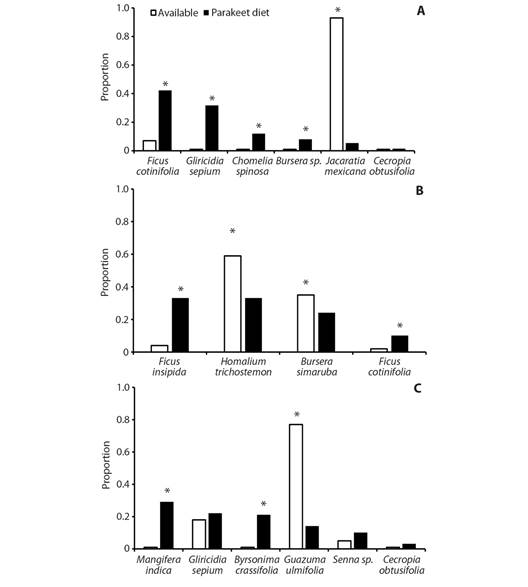
Fig. 2 Proportion of use and availability of food resources for Eupsittula canicularis during the middle to end dry season. A. tropical deciduous forest, B. tropical semi-deciduous forest, C. secondary forest in Santa María Colotepec, Coast of Oaxaca, Mexico. *P < 0.05.
Among the species consumed by the OFP, fruits (syconia) of Ficus insipida and F. cotinifolia showed a highest feeding records and OFP consumed this trees species more than expected from their availability. Ficus has been reported to be importan in the diet of other parrots species during the dry season (Matuzak et al., 2008; Ragusa-Netto, 2002; Ragusa-Netto, 2007; Silva & Melo, 2013; Wermundsen, 1997) and for several tropical frugivorous birds (Bleher et al., 2003). Furthermore, seed of B. simaruba and flowers of G. sepium, and H. trichostemon are also usually eaten by the OFP.
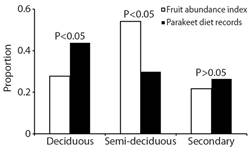
Fig. 3 Proportion of availability (fruiting abundance index) and use of food resources for Eupsittula canicularis during the middle to end dry season in Santa María Colotepec, Coast of Oaxaca, Mexico.
Fruits such as figs are high in water, carbohydrates, and calcium, while seeds are high in protein, minerals, and lipid content (Gilardi, 1996; Jordano, 2000). In addition, the flowers contain essential oil, protein, and carbohydrates (Fonte et al., 2013). In the study area, the fruits of Ficus, seed of B. simaruba and flowers of G. sepium, and H. trichostemon productions play a critical role for providing nutrients during the OFP breeding season. The potential loss of these tree species, as a result of forest degradation, may have consequences in the maintenance of OFP population. Therefore, the knowledge of principal keystone trees for the OFP diet is essential for the forests management decisions, especially in a modified landscape.
Foraging strategies of the Orange-fronted parakeet: The OFP respond to spatial fluctuations in food availability, showing flexibility in the items consumed among forest types. The OFP had a narrower dietary niche in the DF and SDF, while dietary niche is broad in the SF. These results suggest that the OFP has preferences for some resources in primary forests, while in SF, the resources are consumed equally. Similar results have been observed in other psittacine species that can adjust their dietary niche based on abundant resources between habitats (Botero-Delgadillo et al., 2010; Boyes & Perrin, 2010; Matuzak et al., 2008; Renton, 2001). In addition, the OFP selects resources during the breeding season (narrow Hurlbert index), similarly to reported for the Ouvea Island Parakeet Eunymphicus uvaeensis and the Military Macaw A. militaris (Contreras-González et al., 2009; Robinet et al., 2003). These results show that the OFP can modify its foraging strategies by following the food resources available throughout the dry season, consuming certain plant species at the beginning of the breeding season, and including other species at the end. This foraging pattern has been reported in other species of psittacines of the genus Eupsittula and Aratinga (Barros & Marcondes-Machado, 2000; Paranhos et al., 2009; Silva & Melo, 2013; Wermundsen, 1997).
The OFP foraged more frequently in primary forest (DF and SDF) where more resources are available, compared to the SF where the lowest number of feedings parakeets was recorded. These results indicate that the spatial variation in food availability determine foraging strategies and influences habitat use by the OFP inhabit in modified landscape of the Central Pacific, Mexico. Habitat use patterns by the Lilac-crowned Amazon A. finschi are determined by spatial heterogeneity in the availability of food resources (Renton, 2001). Plasticity in food resources use could be advantageous for psittacine that inhabit in modified landscape, where the resources availability is localized in patches of vegetation (Clark & Mangel, 1984).
The present study demonstrates a flexibility in OFP diet according to spatial variations in availability of food resources. The fruits of Ficus species, seed of B. simaruba and flowers of G. sepium, and H. trichostemon could be key resources for OFP during the breeding season. In modified landscape of the Central Pacific, Mexico, it is necessary to maintain the complete forest structure to ensure food resources availability for OFP. The result from this study is an advance on the knowledge of OFP foraging ecology in a modified landscape. Likewise, the information provided in this study is essential for to develop conservation strategies for OFP populations and habitat management decisions.












 uBio
uBio 


