Introduction
Medium (1-15 kg) and large-sized (> 15 kg) mammals are essential for the long-term functioning of terrestrial ecosystems, serving as seed dispersers and top predators, regulating plant populations, and fostering bioturbation (Lacher et al., 2019; McConkey et al., 2012). These animals are particularly diverse in the Neotropics (Burgin et al., 2018; Pillay et al., 2022), where land use intensification (e.g. agriculture and urban expansion) has led to forest fragmentation, isolation, and habitat loss (Tabarelli et al., 2004). These factors, along with illegal hunting, roadkill, and poor farming practices, pose significant challenges to the survival of Neotropical mammals (Alroy, 2017; Bogoni et al., 2020; Crooks et al., 2017; Ripple et al., 2016; Vetter et al., 2011).
Costa Rica occupies 0.03 % of the world's continental area (51 100 km²), but is home to 256 species of mammals, accounting for approximately four percent of the world's mammalian biodiversity (Ramírez-Fernández et al., 2023). This country serves as a corridor for North and South American species, therefore, maintaining habitat connectivity in this region is essential for the long-term survival of Neotropical mammals. Costa Rica is well-known for its conservation efforts, with more than a quarter of its land area under some form of protection (Sistema Nacional de Áreas de Conservación, 2022). Nonetheless, protected areas have become increasingly isolated, with forest patches embedded in agricultural and urban matrices dominating the surrounding landscape (Morera & Sandoval, 2019; Stan & Sánchez-Azofeifa, 2019).
Regional efforts have been implemented to maintain and promote connectivity between protected areas. For instance, the country has been part of the Mesoamerican Biological Corridor since 1997 (Holland, 2012) and of the National Program of Biological Corridors since 2006 (Sistema Nacional de Áreas de Conservación, 2018). However, human activities continue to expand in critical areas such as the Central Valley, where the capital is located, and where forest connectivity is either poor or non-existent.
Tropical forest fragments play a crucial role connecting protected areas, facilitating gene flow, and providing ecosystem services in agricultural and urbanized landscapes (Beca et al., 2017; Coulon et al., 2004; Edwards et al., 2014; Williams et al., 2022). The presence and persistence of mammals in these areas are influenced by the size of the forest patches, their structural characteristics (e. g. floristic composition, successional stage), and resource availability, which varies greatly in seasonal regions (Stoner & Timm, 2011). Therefore, seasonality can be linked to behavioral changes causing mammals to move and actively seek for resources (Stoner & Timm, 2011). This could be crucial in small fragments (< 30 ha) where opportunities to find food, water, and suitable shelters are limited, especially for large-sized mammals (Crooks 2002; Crooks et al., 2017; Morera et al., 2021). For instance, Ocelots (Leopardus pardalis), Greater Grison (Galictis vittata) and Collared Peccaries (Pecari tajacu) expand their home ranges during the dry season (Dillon & Kelly, 2008; Haro-Carrión et al., 2021).
Monitoring mammal presence in fragments within a human-dominated landscape and understanding seasonal variations is essential for ensuring connectivity and conservation of endangered species. Thus, the objectives of this study were to determine the species richness of medium and large-sized mammals in a forest fragment, and to examine if species diversity and detectability varied according to seasonality. Considering that rainfall patterns and tree phenology vary drastically in the studied location, we hypothesized that seasonality affected these animals' diversity and detectability. Since there are more resources available during the wet season (e. g., fruits, insects, and water) (Basset et al., 2015; da Silva et al., 2011; Darosci et al., 2021; Valenzuela & Ceballos 2000), we expected a higher diversity and number of detections of medium and large-sized mammals during this season. However, we expected to find a higher species richness during the dry season, when predators and other large mammals may be detected when traveling long distances in search of resources.
Materials and methods
Study site: We carried out this study in the Municipal Forest of Atenas (MFA) Anselmo Murillo Jiménez, a 26.4 ha premontane moist forest fragment, located in the province of Alajuela, in the Central Valley of Costa Rica (950-1 060 m.a.s.l.; Fig. 1). This area was previously used for cattle ranching, coffee, and sugar cane plantations, until the 1930s, when the Municipality of Atenas purchased the property to protect it as a source of drinking water for neighboring communities. Currently, this forest is part of the Montes del Aguacate Biological Corridor, and it is co-managed by the Municipality of Atenas, the community development association of Alto del Monte and the Administrative Association for Aqueducts and Sewers of Plancillo, providing drinking water to about 1 800 people as well as serving as a recreational area.
The forest is surrounded mainly by shade coffee plantations, Highway 3 and riparian forest (Avalos et al., 2006), and the nearest large, forested areas (> 2 000 ha) are about 25 km away. The MFA maintains approximately 119 tree species, the most abundant being the densely flowered Inga or ''guabo salado'' (Inga densiflora), the invasive species Syzygium jambos (known as Rose Apple), and secondary successional species such as the ''espavel'' (Anacardium excelsum), the kapok tree (Ceiba pentandra), and Luehea seemannii (Avalos et al., 2006; Brassil et al., 2000). Atenas has a strong seasonality, with the rainy season lasting from May to November, and receiving an averaging annual precipitation of 3 000 mm. During the dry season (December to April), seasonal streams in the MFA dry out for 2-3 months. Permanent springs are used as sources of drinking water.
Data collection: We established 26 camera trap stations throughout the MFA (Fig. 1). From August 2021 to April 2022, one camera trap (Blazevideo SL112) was active at each station for 12-21 days throughout each season (dry and wet). We placed 18 cameras near human-made trails, four cameras off trails (> 20 m away) and four near streams, aiming to detect species that avoid trails or that move along streams.
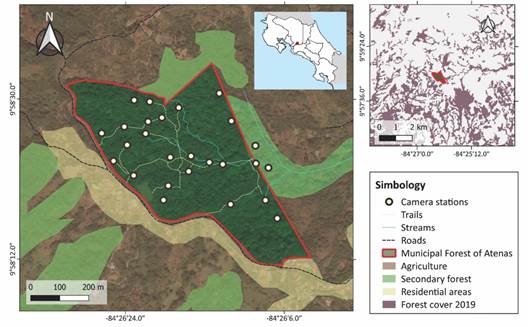
Fig. 1 Distribution of camera trap stations to survey medium (1-15 kg) and large-sized mammals (> 15 kg) at the Municipal Forest of Atenas (Alajuela, Costa Rica), between August 2021 and April 2022. Sources: Google Satellite 2022, Centro Nacional de Alta Tecnología, 2022.
Each site was separated at least 50 m from the nearest site. Cameras were set to record ten second videos, with a five second interval before resetting the trigger, and were active 24 hours a day. We strapped each camera to a tree at approximately 0.5 m above the ground and recorded its location with a GPS (Garmin etrex 10). We also accounted for direct observations in the field and the use of three camera traps placed in the canopy for a one month of exploratory and aleatory sampling. These cameras were placed approximately 12 m above the ground and were active between March and April 2022, for a total of 89 camera trap days.
Data analysis: We used digiKam (version 7.3.0) (digiKam Developers Team, 2020) to tag each video with the observed species. We used ExifTool (version 12.3.2.0) (Harvey, 2022) and the package camtrapr (Niedballa et al., 2016) in R (version 4.2.0) (R Development Core Team, 2022) to extract the metadata. Delta time was set to 120 minutes so that detections of the same species within a 120-minute timespan would be considered as one detection. Considering the small study area (26.4 ha) and the home range of the medium and large-sized mammals (from ~ 2 ha for Cuniculus paca to 2 600 ha for Leopardus pardalis), a detection was not necessarily an independent detection (Beck-King et al., 1999; Dillon & Kelly, 2008). Frequency of detection for each species was calculated as the number of detections per camera trap day.
We characterized mammal's species diversity by applying rarefaction and extrapolation curves using Hill numbers for sample-based incidence (Chao et al., 2014; Hsieh et al., 2016). We used species detectability (number of detections per species per camera trap day) as the relative incidence when performing the rarefaction/extrapolation curves. Hill numbers represent the effective number of species within a community (Jost, 2006). The first Hill number (q0) is equivalent to species richness. The second one (q1) corresponds to the effective number of equally common species. The third one (q2) includes detectability as well, but it prioritizes the species with the higher frequency of detections (Chao et al., 2014; Hsieh et al., 2016).
We applied the same analysis to compare dry and wet season mammal diversity, considering the confidence interval (95 %) around the rarefaction and extrapolation curves. We compared the curves to test the hypothesis that seasonality affected diversity. We also compared the average number of detection/camera trap day between seasons using a Wilcoxon test. Analyses were performed using R, and the package iNEXT (Hsieh et al., 2016). Data from direct observations and the canopy camera traps were not included in these analyses.
Results
Medium and large-sized mammals of the MFA: We registered 19 species of medium and large-sized mammals distributed in 12 families and five orders (Table 1, Fig. 2.) (Hereafter we refer to species detected by their common names).

Fig. 2 Medium (1-15 kg) and large-sized (> 15 kg) mammal species classified as ''endangered'' in Costa Rica (Ministerio de Ambiente y Energía, 2017), detected at the Municipal Forest of Atenas (Alajuela, Costa Rica). A. Puma (Puma concolor). B. Ocelot (Leopardus pardalis). C. Jaguarundi (Herpailurus yagouaroundi). D. Neotropical River Otter (Lontra longicaudis).
Fifteen species were recorded using the camera traps in the understory, whereas we recorded four species by direct observations and by using camera traps in the canopy (Table 1). Understory camera traps were active for 810 camera days. We registered 557 detections. The general frequency of detections was 0.688 detections/camera trap day (mean = 0.046 ± 0.073 detections/camera trap day). The average number of species detected per station was 3.14 ± 1.21. Dominant species in the medium and large-sized mammals assemblage included the White-nosed Coati (0.254 detections/camera trap day), the Central American Agouti (0.163 detections/camera trap day), the Lowland Paca (0.139 detections/camera trap day), the Common Opossum (0.069 detections/camera trap day), and the Northern Raccoon (0.059 detections/camera trap day) (Fig. 3).
In the rarefaction/extrapolation curve, the effective number of common (q1 = 6) and dominant species (q2 = 4) reached an asymptote (Fig. 4A).
Table 1 Medium (1-15 kg) and large mammals (> 15 kg) recorded at the Municipal Forest of Atenas (Alajuela, Costa Rica) between August 2021 and April 2022.
| Family | Species | Common name | Guild | IUCN | MINAE |
| Mustelidae | Eira barbara (Linnaeus 1758) | Tayra | O | Least concern | Least concern |
| Galictis vittata (Schreber 1776)* | Greater Grison | C | Least concern | Vulnerable | |
| Lontra longicaudis (Olfers 1818) | Neotropical River Otter | C | Near threatened | Endangered | |
| Felidae | Leopardus pardalis (Linnaeus 1758) | Ocelot | C | Least concern | Endangered |
| Puma concolor (Linnaeus 1771) | Puma | C | Least concern | Endangered | |
| Herpailurus yagouaroundi (É. Geoffroy Saint-Hilaire 1803) | Jaguarundi | C | Least concern | Endangered | |
| Procyonidae | Nasua narica (Linnaeus 1766) | White-nosed Coati | O | Least concern | Least concern |
| Procyon lotor (Linnaeus 1758) | Northern Raccoon | O | Least concern | Least concern | |
| Potos flavus (Schreber 1774)** | Kinkajou | O | Least concern | Least concern | |
| Canidae | Canis latrans (Say 1823) | Coyote | O | Least concern | Least concern |
| Urocyon cinereoargenteus (Schreber 1775) | Gray Fox | O | Least concern | Least concern | |
| Mephitidae | Conepatus semistriatus (Boddaert 1785) | Striped Hog-nosed Skunk | C | Least concern | Least concern |
| Cuniculidae | Cuniculus paca (Linnaeus 1766) | Lowland Paca | H | Least concern | Vulnerable |
| Dasyproctidae | Dasyprocta punctata (Gray 1842) | Central American Agouti | H | Least concern | Least concern |
| Erethizonthidae | Coendou mexicanus (Kerr 1792)** | Mexican Hairy Dwarf Porcupine | H | Least concern | Least concern |
| Dasypodidae | Dasypus novemcinctus (Linnaeus 1758) | Nine-banded Armadillo | I | Least concern | Least concern |
| Cebidae | Cebus imitator (Thomas 1903)** | Panamanian White-faced Capuchin | O | Vulnerable | Least concern |
| Myrmecophagidae | Tamandua mexicana (Saussure 1860) | Northern Tamandua | I | Least concern | Least concern |
| Didelphidae | Didelphis marsupialis (Linnaeus 1758) | Common Opossum | O | Least concern | Least concern |
Guild: O = Omnivore, Carnivore, H = Herbivore, I = Insectivore. IUCN = International Union for Conservation of Nature's Red List of Threatened Species (https://www.iucnredlist.org/). MINAE = National Ministry of Environment and Energy, Costa Rica (Ministerio de Ambiente y Energía, 2017). *Species detected by observation. **Species detected by camera traps placed in the canopy (12 m above the ground).
However, because some uncommon species have not yet been detected, the species richness (q0 = 15) could increase slightly with additional sampling effort (Fig. 4A). We have already recorded the following rare species: Jaguarundi (0.001 detections/camera trap day), Puma (0.003 detections/camera trap day), Neotropical River Otter (0.004 detections/camera trap day) and Gray Fox (0.003 detections/camera trap day). Except for the Gray Fox, all of them are listed as endangered species in Costa Rica (Table 1).
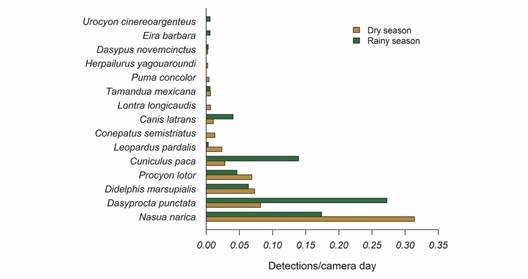
Fig. 3 Frequency of detections of medium (1-15 kg) and large-sized mammals (> 15 kg) at the Municipal Forest of Atenas (Alajuela, Costa Rica) during the wet (August-November 2021) and dry season (December 2021 - April 2022).
Seasonality and mammal diversity in the MFA: The sampling effort during the dry season consisted of 465 camera days and 295 detections, which accounted for 86.6 % of the reported mammals in the MFA. The sampling effort during the wet season was 345 camera days and 262 mammal detections, with 73.3 % of the mammals detected in this study (Fig. 3). Detection frequencies were higher during the wet season (Dry season = 0.042 ± 0.080 detections/camera trap day; Wet season = 0.051 ± 0.082 detections/camera trap day; W = 417, P = 0.04).
Seasonality influenced the dominating species (q2) but did not affect species richness (q0) or the number of effective common species (q1) (Fig. 4). However, if sampling effort is increased, the overall species richness may also increase (Fig. 4A). Between the dry and wet seasons, the identity and prevalence of dominant species varied little (Fig. 3). The most frequent species recorded during the dry season was the White-nosed Coati (0.314 detections/camera trap day), while the most frequent mammal during the wet season was the Central American Agouti (0.273 detections/camera trap day) (Fig. 3). During the dry season, there were more detections of the Lowland Paca (Fig. 3).
The presence of rare species was the main difference between wet and dry seasons. The Striped Hog-nosed Skunk, the Puma, the Neotropical River Otter, and the Jaguarundi were only observed during the dry season.
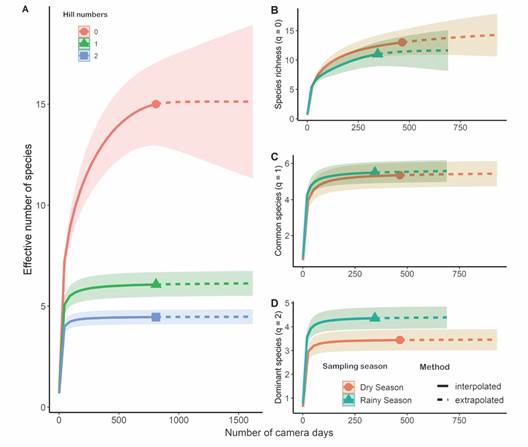
Fig. 4 Rarefaction and extrapolation curves for the medium (1-15 kg) and large-sized (> 15 kg) mammals detected at the Municipal Forest of Atenas (Alajuela, Costa Rica). A. Effective number of species using the whole sampling period and the three first orders of the Hill numbers. B. Effective number of species, C. Effective number of common species and D. Effective number of dominant species, all of them comparing wet and dry seasons.
The Tayra and the Gray Fox were exclusively detected during the wet season. Except for the Striped Hog-nosed Skunk (0.013 detections/camera trap day), all of them had frequencies lower than 0.007 detections each camera day (Fig. 3). Despite these variations, because they are rare species, they could have been detected in both seasons with an increased sampling effort.
Discussion
Medium and large-sized mammals of the MFA: We detected 19 species of medium and large-sized mammals at the MFA. Compared to similar studies (Botelho et al., 2012; Michalski & Peres, 2007), we found a relatively high richness given that this forest fragment is only 26.4 ha and is primarily surrounded by agricultural fields and forest fragments. It is known that there is a tolerance gradient to forest-cover change, ranging from species that are highly sensitive to fragmentation to species that are tolerant or that even benefit from it (Garmendia et al., 2013). For instance, Jaguars (Panthera onca) or White-lipped Peccaries (Tayassu pecari) are highly sensitive to habitat loss and prefer continuous forest, whereas species such as the White-nosed Coati, the Common Opossum and the Northern Raccoon are known to be species that adapt easily to fragmented environments (Daily et al., 2003; Garmendia et al., 2013).
Central American Agoutis and Lowland Pacas are common rodents in fragmented landscapes. These animals are considered important seed predators and dispersers. Agoutis are primarily diurnal and are commonly found in human altered landscapes, including agroforestry systems such as coffee plantations (Sánchez-Brenes & Monge, 2021), which is a common land use at the studied site. Pacas are nocturnal animals which are considered vulnerable in Costa Rica, due to heavy hunting pressure, which although illegal, is a common practice in the study site. The high number of detections of these species, while not necessarily related to their abundance, is a good indicator of prey availability for predators such as coyotes, ocelots and pumas (Emsens et al., 2014).
Although small fragments (< 30 ha) are unlikely to support a viable population of large predators such as pumas, there is evidence that they may be crucial to facilitate their mobility, providing the connectivity that is critical to sustain viable populations, since these patches are used as ''stepping stones'' between larger protected areas (Dalecky et al., 2002; Garmendia et al., 2013; Sampaio et al., 2010). Population viability also depends on habitat matrix surrounding forest fragments (Garmendia et al., 2013). Agroforestry systems are known to increase connectivity across altered landscapes, providing habitat for generalist species and facilitating animal movements, especially those of forest-dependent species (Perfecto & Vandermeer, 2008).
The study area is surrounded by a complex matrix that includes residential areas, secondary forest patches, and agricultural sites dominated by shade coffee plantations and pasturelands. Shade trees in these coffee plantations are capable of natural regeneration (Häger et al., 2015), which may facilitate connectivity among forest patches. Previous studies have shown that larger patches surrounded by secondary forests and tree crops maintain a higher number of species of medium and large-sized terrestrial mammals (Garmendia et al., 2013). These landscape characteristics may explain the relatively high mammal richness observed at the MFA, which can be considered as a refuge for threatened species and an important transit area for large mammals.
Seasonality and mammal diversity in the MFA: As we hypothesized, detectability was higher during the wet season, when more resources are available (Basset et al., 2015; da Silva et al., 2011; Darosci et al., 2021; Valenzuela & Ceballos 2000). We also observed a seasonal turnover in species, which may be related to food habits and available resources. For instance, herbivores such as the Central American Agouti and the Lowland Paca were detected up to three times more frequently during the wet season, while the White-nosed Coati, the Northern Raccoon and the Ocelot increased their detectability during the dry season (Fig. 3). This pattern can be related to changes across space and time that generates a greater heterogeneity of micro-habitats inside a small forest fragment (Basset et al., 2015; Haro-Carrión et al., 2021; Valenzuela & Macdonald, 2002).
Seasonality and rainfall pattern fluctuations directly affect plant phenology and therefore, fruit availability. The densely flowered Inga (I. densiflora) is one of the most common trees at the MFA (Silva-Forsberg, 2008). This species fructifies during the wet season (Koptur, 1983) and is eaten by the Central American Agouti (Sánchez-Brenes & Monge, 2021). Along with I. densiflora fruits, other common trees, like the introduced Rose Apple (S. jambos), and the shrub Allophylus occidentalis, whose fructification period also coincides with the rainy season, may be linked to the high detectability of herbivores at this time of year (Avalos et al., 2006; Gargiullo et al., 2008; Patel et al., 2017). On the other hand, it is well known that ocelot home-range size is larger during the dry season when food is thought to be scarcer (Dillon & Kelly, 2008). Since a small forest fragment cannot support a viable feline population, detectability of these carnivores depends on the use of corridors for movement (Morera et al., 2021; Salom-Pérez et al., 2022).
Finally, the White-nosed Coati was one of the most frequently detected species along the sampling period, but during the dry season it represented 50 % of the detections, overshadowing other common species such as the Central American Agouti and the Lowland Paca that were dominant during the rainy season. As this species tends to have a larger home-range and be more widely dispersed on the area during the dry season, this could be associated to a greater detection in the MFA during this season, where we were able to record at least 40-50 individuals in the same band (Valenzuela & Ceballos, 2000; Valenzuela & Macdonald, 2002). This pattern may also be connected to the coati's parental care behavior, as females tend to stay longer in a refuge area caring for the young, and thus they are less mobile during the wet season (Valenzuela & Ceballos, 2000).
Implications for water management and mammal conservation: Although, most of the mammalian species we registered are classified as ''least concern'' by the IUCN red list, in Costa Rica two of these species are considered vulnerable (Lowland Paca and Greater Grison) and four are endangered (Puma, Jaguarundi, Ocelot and Neotropical River Otter). The presence of these species in a relatively small forest fragment (< 30 ha) surrounded by an agricultural matrix, highlights the importance of the MFA in the protection and connectivity of species, contributing to gene flow between isolated populations. Despite its importance, the MFA lacks a management plan. Information presented in this document contributes to a better understanding of mammal ecology, the implementation of proper forest management strategies, and the strengthening of community organization capacities centered around water preservation.
Like the MFA, in Costa Rica there are about 1 400 local associations that manage forest fragments that provide water to about 25.5 % of the population (1.1 million people) (Instituto Costarricense de Acueductos y Alcantarillados, 2016). Assessment of the water sources and monitoring of the area around springs are therefore essential for efficient water management. Due to their sensitivity to human interference, medium and large-sized mammals serve as powerful bioindicators and an important tool to assess the integrity of those forests (Cove et al., 2013; Salom-Pérez et al., 2021). It is critical to examine the relationship between mammal conservation and water protection. Conservation efforts should be multipurpose while also showing the tangible benefits of protecting forested areas in the provision of critical environmental services. This study generates baseline information that shows how protecting forest fragments to secure drinking water also helps to restore and maintain stable animal communities.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


