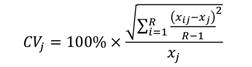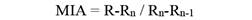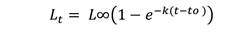Introduction
Rita rita (Hamilton, 1822) belongs to the family Bagridae, which is distributed in the freshwaters of India as well as many other countries in the Asian continent. The target fish species has high market value on account of its consumer preference, nutritional value, ornamental value and palatability (Gupta & Banerjee, 2014; Mohanty et al., 2015). There has been only one report (Tamubi et al., 1990) on the age and growth characteristics of this species, wherein no consideration of precision of age estimates was taken into account. Molur and Walker (1998) categorised it as a threatened fish of the Indian subcontinent and suggested that it could face the risk of extinction mainly due to overfishing and loss of breeding sites. Bangladesh, The International Union for Conservation of Nature (IUCN) (2015) categorizes fish as endangered in Bangladesh but globally as Least Concern. Therefore, the population of Rita rita should be scientifically managed and protected by undertaking suitable measures.
Age estimation of fish is a necessary first step in age-based fish population assessment and successful resource management (Maceina & Sammons, 2006). The ability to obtain precise age estimates is critical for estimating dynamic rate functions like mortality and recruitment (Khan & Khan, 2020). A precision measure is important for evaluating the relative simplicity of determining the age of a given hard structure, examining the reliability of an individual's age estimations, and measuring the ager's level of skill relative to others (Campana, 2001). By incorporating a validation method, errors in estimating the age of fish can be minimized. There is an alternative validation technique i.e., comparing the age estimates between different hard anatomical structures that potentially provide valuable insights into the accuracy and inaccuracy of age-estimating structures (Sylvester & Berry Jr, 2006).
Researchers have compared age enumerated from different hard structures in freshwater fishes such as Channa punctata (Khan et al., 2013), Labeo bata (Khan et al., 2015), C. striata (Khan et al., 2017), Sperata aor (Nazir & Khan, 2020), Leuciscus vorax (Rashid & Basusta, 2021), Cycleptus elongatus (Radford et al., 2021), Salvelinus namaycush (Osborne et al., 2022) to quantify precision and detect biases related to each structure.
Amongst several methods available to validate fish age interpretations (Campana, 2001), Marginal Increment Analysis (MIA) is one of the extensively utilized methods (Campana, 2001). This technique is based on the notion that growth rings are produced annually, and the width of the outer ring will depict an annual sinusoidal cycle if plotted against the month of capture. (Campana, 2001; Okamura et al., 2013).
Age estimation in several fish species has been validated using MIA for example, Merluccius hubbsi (Costa et al., 2018); Brachyplatystoma rousseauxii (Hauser et al., 2018). Several studies have successfully used the von Bertalanffy Growth Function model (VBGF) to estimate growth in a variety of fish species, such as Schizopyge niger, S. curvifrons and Schizothorax esocinus (Sabah & Khan, 2014), Channa punctata (Khan et al., 2019), Gerres subfasciatus (Gray, 2019), Catla catla (Brraich & Kaur, 2022). The basic biological information required to devise scientifically sound management policies for the Rita rita population remains warranted, particularly those involving precise age estimation and growth trends under changing conditions across all of the fish's primary habitats. Otoliths have previously been used to investigate age and growth studies in Rita rita (Tamubi et al., 1990) however, there is no information available on the precision of its hard structure for age estimation.
Therefore, this study was undertaken to assess age estimates derived from various hard (calcified) structures (vertebrae, sectioned otoliths, whole otoliths, opercular bones and pectoral spines) to identify the best structure determining accurate age estimate in Rita rita; the annulus formation was validated using MIA, and the VBGF equation was developed using length-at-age data from the structure giving precise age estimate.
Materials and methods
Sample collection: A total of 390 fish samples of Rita rita were collected on a monthly basis during the period September 2018 to August 2019 from Narora site (28°19'68'' N & 78°38'14'' E) of River Ganga, Mathura site (27°49'24'' N & 77°67'37'' E) of River Yamuna and Moradabad site (28°83'86'' N & 78°77'33'' E) of River Ramganga (Fig. 1). Fish identification was done according to Talwar and Jhingran (1991). To the nearest centimeter, the total length (TL) was measured. The total weight (TW), which includes the gut and gonads, was measured to the nearest gram. Otoliths, opercular bones, vertebrae and pectoral spines were extracted from the fish and prepared for ageing according to the protocols standardized by Fish biology and Otolith Research Laboratory (Khan & Khan, 2009; Khan et al., 2013; Khan et al., 2017; Sabah & Khan, 2014). Otoliths were sectioned according to Khan et al. (2016). For sectioning, the other otolith of a pair was embedded in silicon moulds consisting of (Epothin TM Epoxy Resin and Epothin® Epoxy Hardener in a 5:2 ratio) which were lightly coated with release agent and allowed to solidify overnight for the media to harden. Otoliths were sectioned by using IsoMet® Low Speed Saw (Buehler, 41 Waukegan Road, Lake Bluff, Illinois-60044, USA) and after that each section was polished using 1 200-grit CarbiMet® 2 Abrasive Discs on an otolith polisher, which has been designed and developed in Fish biology and Otolith Research Laboratory. A Nikon® SMZ745T stereo zoom microscope was used to analyze all the ageing structures. Two readers separately analyzed each calcified structure. A year's growth was defined as an opaque and translucent zone combined; age was defined by the number of translucent zones.
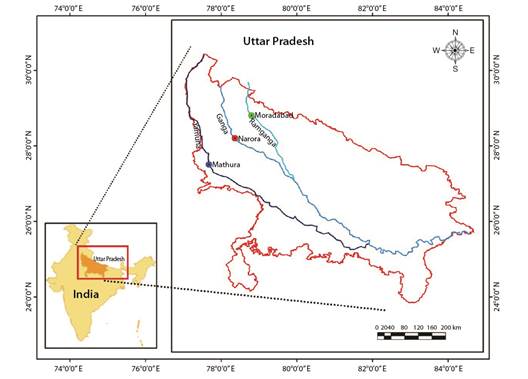
Fig. 1 Map showing the collection sites of Rita rita from the River Ganga and its tributaries: River Yamuna and River Ramganga.
Statistical analysis and calculations: The average per cent error (APE), coefficient of variation (CV), and per cent agreement (PA) between the readers and the pair of ageing structures were calculated to compare age estimates from different hard anatomical structures. APE was calculated as per the formula given by Beamish and Fournier (1981).
Where in xij represents the ith age estimation of the jth fish, xj refers to the computed mean age for the jth fish, and R denotes the frequency of ageing for each fish.
The coefficient of variation was determined as the ratio of standard deviation over the mean (Campana, 2001):
Where CV j is the coefficient of variation for the j th fish.
Campana (2001) suggested APE and CV to be reliable parameters for precise age estimation in fish. While determining the most suitable method for precise age estimation, PA has been used as one of the precision parameters (Sabah & Khan, 2014). The percentage of observations having similar age estimates divided by the total number of observations on age estimates is defined as percent agreement. Percent agreement was calculated using the template for ageing precision (Sutherland, 2006). A post- hoc test, Duncan's Multiple Range Test (Gomez & Gomez, 1984), was used to determine variations in mean age readings from different ageing structures (Khan & Khan, 2009).
Marginal Increment Analysis: Annulus formation on vertebrae was validated by Marginal Increment Analysis using the equation:
R is the otolith radius, Rn is the last complete growth ring radius, and Rn-1 is the previously completed growth ring radius. Image analysis software was used to take measurements from the focus to the outer edge of each growth ring (Rn). To demonstrate periodic trends in annulus development, monthly mean MIR values were plotted (Lessa et al., 2006).
Growth analysis: To evaluate variability in growth, length-at-age data collected from vertebrae (the most appropriate ageing structure in Rita rita) was used to develop the VBGF equation (Ricker, 1975) by using non-linear least-squares regression:
Where, Lt= total length (cm) of fish at age t; L∞ = asymptotic mean length; k = rate constant that determines the rate at which Lt approaches L∞; t = time or age of the fish; t0 = the hypothetical age at which the fish had zero length.
Comparison between observed and calculated mean length-at-age was verified with student's t-test (Zar, 1996) for the selected fish species. Growth parameters were estimated using the non-linear regression method in a Microsoft Excel-based application developed by Cope and Punt (2007).
Results
The sample size, minimum and maximum total length, used in the ageing precision for the selected fish species are presented in Table 1. The vertebrae in Rita rita provided a precise age estimate. It exhibited the least APE (1.54 %) and CV (3.17 %) values followed by sectioned otoliths, whole otoliths, opercular bones and sectioned pectoral spines (Table 2). The vertebrae had the highest percentage of reader agreement (Table 2). A comparison of vertebrae age estimates was analyzed with those from other structures (i.e., sectioned otoliths, opercular bone, whole otoliths and sectioned pectoral spines) suggested the least variation between vertebrae vs sectioned otoliths age estimates followed by vertebrae vs opercular bone, vertebrae vs whole otoliths and vertebrae vs sectioned pectoral spine (Table 2).
Table 1 Estimated parameters of Rita rita collected from the selected rivers.
| Rivers | Sample size | TL Range (cm) | Mean (cm) |
| Ganga | 140 | 9.3 - 57.2 | 36.4 |
| Yamuna | 120 | 10.2 - 56.1 | 33.6 |
| Ramganga | 130 | 9.8 - 54.4 | 34.2 |
TL: Total length.
Table 2 Measures of precision in Rita rita.
| Ageing structures | APE | CV | PA |
| Vertebrae | 1.54 | 3.17 | 88.3 |
| Sectioned otoliths | 1.61 | 3.51 | 86.8 |
| Whole otoliths | 2.06 | 4.10 | 84.6 |
| Opercular bones | 3.38 | 6.12 | 80.2 |
| Pectoral spines | 5.40 | 8.44 | 76.3 |
| Between structures | |||
| Vertebrae- sectioned otoliths | 1.66 | 3.38 | 87.2 |
| Vertebrae - whole otoliths | 2.00 | 3.83 | 85.4 |
| Vertebrae - opercular bones | 3.53 | 5.65 | 81.9 |
| Vertebrae-pectoral spines | 5.93 | 7.52 | 79.0 |
PA: Percentage of Agreement, APE: Average Percentage Error, and CV: Coefficient of Variation (CV).
Mean age readings from vertebrae were significantly (P < 0.05) different from pectoral spines but similar (P > 0.05) to the age estimates from sectioned otoliths, whole otoliths and opercular bones. Thus, in the absence of the most appropriate age estimate (vertebrae), we may use age data from sectioned otoliths, whole otoliths and opercular bones but the use of pectoral spines may be avoided to the maximum possible extent (Table 3).
Table 3 Comparison of mean values of age estimates from different hard structures in Rita rita.
| Ageing Structures | Mean values of age estimates |
| Vertebrae | 4.4223b |
| Sectioned otoliths | 4.2896b |
| Whole otoliths | 3.9918ab |
| Opercular bones | 3.1854ab |
| Pectoral spines | 2.876a |
*Values having similar superscripts are insignificantly different (P > 0.05) from each other.
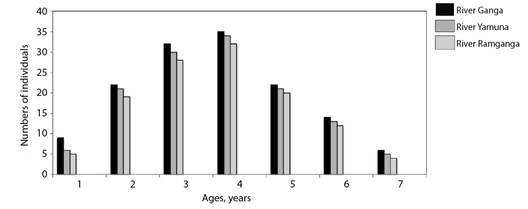
The von Bertalanffy growth model was fit using the ages estimated from vertebrae. The von Bertalanffy growth parameters of Rita rita from the three rivers are presented in Table 4. Readability scores presented in Table 5 showed that vertebrae had the highest readability score (64.1) followed by sectioned otoliths (56.41), whole otoliths (41), opercular bones (14.36) and sectioned pectoral spines (2.05). Fig. 2 shows the number of specimens in each age group in all the studied rivers. The age group 3 and 4 were most dominant in the sampling from all three rivers while a smaller number of samples were found in age group 7. Ageing structures in Rita rita vertebrae, sectioned otoliths, whole otoliths, opercular bones, and sectioned pectoral spines are shown in Fig. 3. Age bias graphs between vertebral age estimations and each of the other ageing structures are shown in Fig. 4.
Table 4 von Bertalanffy growth parameters in Rita rita collected from the Gangetic River System.
| Species | Selected rivers | Sampling sites | Ageing structures used | L∞ (cm) | k | t 0 | Max. Age (years) |
| Rita rita | Ganga | Narora | Vertebrae | 90.19 | 0.145 | 0.51 | 7 |
| Rita rita | Yamuna | Mathura | Vertebrae | 91.19 | 0.14 | 0.59 | 7 |
| Rita rita | Ramganga | Moradabad | Vertebrae | 89.63 | 0.15 | 0.61 | 7 |
Table 5 Distribution of readability scores for different hard structures in Rita rita.
| Readability score | 1 | 2 | 3 | 4 | 5 |
| Vertebrae | 7.69 | 64.1 | 15.38 | 8.97 | 3.85 |
| Sectioned otoliths | 5.64 | 56.41 | 16.4 | 12.31 | 9.23 |
| Whole otoliths | 3.08 | 41 | 11.03 | 18.97 | 25.9 |
| Opercular bones | 1.79 | 14.36 | 4.62 | 30.77 | 48.46 |
| Sectioned pectoral spines | 2.05 | 6.15 | 40 | 51.79 |
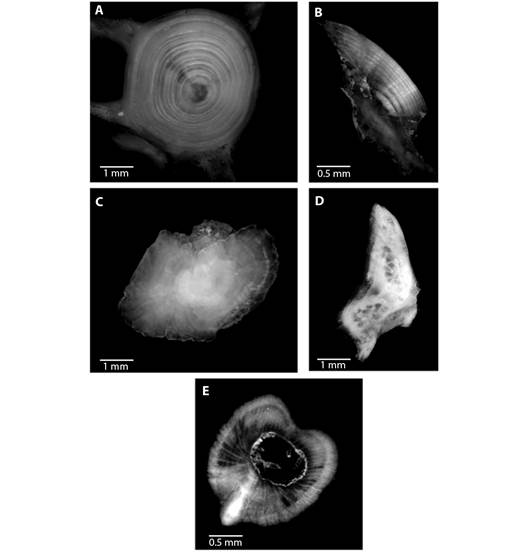
Fig. 3 Ageing structures in Rita rita A. vertebrae, B. sectioned otoliths, C. whole otoliths, D. opercular bones and E. sectioned pectoral spines. Scale bar: 1 mm.
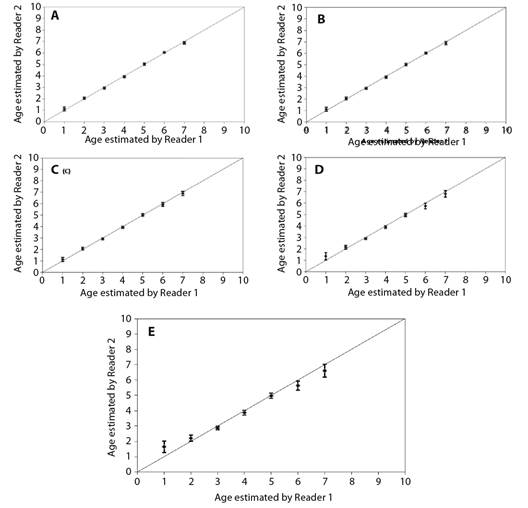
Fig. 4 Age bias graphs for Rita rita between two independent readers: A. vertebrae, B. sectioned otoliths, C. whole otoliths, D. opercular bones and E. pectoral spines. Each error bar represents the 95 % confidence interval, and the solid line indicates the theoretical 1:1 agreement line of age estimates between readers. Points above the line indicate ages that were overestimated, whereas points below the line indicate ages that were underestimated.
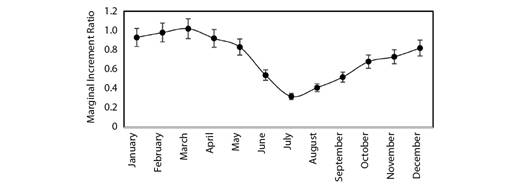
Marginal Increment Analysis has been used to validate age estimation in the target fish species. The monthly mean of Marginal Increment Ratio (MIR) declined from a highest of 1.02 in March to the lowest of 0.32 in July (Fig. 5). The results of MIR revealed that the annulus formation occurred from May to September (Fig. 5). The proportion of opaque bands on vertebrae increased regularly from August to March and then decreased from April to July. These results suggest that the narrow translucent band formed during the monsoon season (May to September) and the wide opaque band formed during the rest of the months.
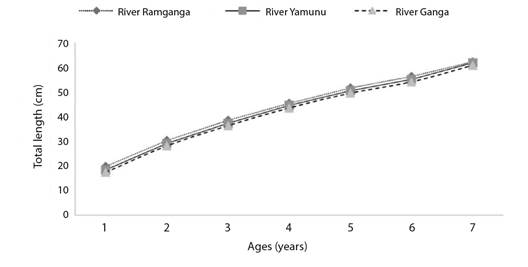
The von Bertalanffy growth function equations derived using length at age data of vertebrae of Rita rita were Lt = 90.19(1-e-0.145(t+0.51)) for River Ganga; Lt = 91.19 (1-e-0.14(t+0.59)) for River Yamuna; Lt = 89.63 (1-e-0.15(t+0.68)) for River Ramganga. There were no significant variations between Rita rita's calculated and observed lengths of the River Ganga (R2 = 0.9781; d.f. = 6, P > 0.05), River Yamuna (R2 = 0.9686; d.f. = 6, P > 0.05) and River Ramganga (R2 = 0.9731; d.f. = 6, P > 0.05) The von Bertalanffy growth curves of Rita rita from the three rivers: River Ganga, River Yamuna and River Ramganga showed significant differences (P < 0.05) with each other (Fig. 6).
Discussion
In this study, the vertebrae of Rita rita showed regularly formed annual rings, which were clear to count, compared to other hard anatomical structures (whole otoliths, sectioned otoliths, opercular bones, pectoral spines). Our results corroborate with the findings of Li and Xie (2008) that in Siluriformes fishes, the best ageing structure is vertebrae that were easy to process and exhibited regularly formed clear rings. Precise age estimation using vertebrae has been reported in a variety of freshwater fishes, such as Heteropneustes fossilis (Khan et al., 2013), Schizothorax esocinus (Sabah & Khan, 2014), Mastacembelus armatus, and Ompok pabda (Khan et al., 2015), Capoeta banarescui (Bostanci et al., 2015), S. aor (Nazir & Khan, 2020).
Next to vertebrae, otoliths provided better age estimates than other ageing structures. Sectioned otoliths after polishing exhibited clearer annuli as compared to whole otoliths and thereby requiring less effort and time by the readers to estimate age. Several studies reported sectioned otoliths to provide precise age estimates in Paralichthys dentatus (Sipe & Chittenden, 2001), C. striata (Khan et al., 2017). Because of its distinctive attributes of being metabolically inert, otoliths are usually utilized as an ageing structure, not showing reabsorption and exhibiting acellular growth throughout fish life. (Phelps et al., 2007).
Whole otoliths in Rita rita did not show distinct annuli because whole otoliths are thick at the center which reduces the clarity of growth lines. As a result, the opaque rings on otoliths could not be distinguished clearly. Since fish growth shows declining trends with age, the annuli come closer to each other near the edges of the ageing structures and hence it becomes difficult to distinguish annuli in small and thick whole otoliths used in this study. Whole otoliths underestimated the fish age compared to vertebrae, opercular bones, and sectioned otoliths. There are several findings in support of our study that the use of whole otoliths rather than sectioned otoliths can lead to an underestimation of fish age (Abecasis et al., 2006; Khan et al., 2016; Khan et al., 2017).
Khan et al. (2016) reported that the use of the whole otolith in S. aor may underestimate the age because growth rings in older individuals were not clear enough to read because of curved edges, and the zones across the edges became dense and thus harder to distinguish the growth rings. In the current study, opercular bones were found to be inferior to other calcified structures (vertebrae, sectioned otoliths, and whole otoliths) to age Rita rita. It was very difficult to assess the age of the fish using opercular bones because annual rings were poorly defined and irreconcilable.
According to several studies, opercular bones may overlook the fish age due to its thickness at the base and this thickening continues into spongy tissue, making the very first and/or subsequent annuli less noticeable. (Ma et al., 2011; Zhiming et al., 2018). Opercular bones were found to be less accurate in a variety of fish species when compared to other structures such as scales and otoliths in Labeo rohita and C. marulius (Khan & Khan, 2009), otoliths and vertebrae in Schizothorax oconnori (Ma et al., 2011).
In this study, pectoral spines showed less clarity to identify and read annuli as compared to other ageing structures in Rita rita. They had the minimum PA and maximum APE and CV values among readers. Our results corroborated with those of previous reports since the pectoral spine continually overlooks the age of the fish because of its nucleus that might be absorbed and replaced by a hole (vascularization). As fish age, its central lumen gets enlarged because of the increased number of vascularized tissues and its structure changes with age, thus eroding early growth increments (McFarlane & King, 2001). If initial growth rings were not identified correctly then it leads to an underestimation of the age and thereby causes an overestimation of growth and natural mortality coefficients, which can have serious consequences for fish stock management and policy-making (Casey & Natanson, 1992). Our results are in agreement with other studies that have noted complications in the analyses of annuli working with the pectoral spines in fishes for example Ictalurus punctatus and Pylodictis olivaris (Crumpton et al., 1987), and S. aor (Nazir & Khan, 2020).
Validation of the age estimates in Rita rita was done by MIA on vertebrae. There are several causes of annuli formation in fish such as water temperature, light exposure, metabolism, endocrine, feeding, reproduction and the environment (Morales-Nin & Panfili, 2005). Spawning seems to be related to the formation of growth checks on the vertebrae of Rita rita. the mating season in Rita rita is from May to September, with spawning maxima in June and September in River Ganga which appears to be coincident with the annulus formation as shown in this study (Alam et al., 2016).
Slow-growing species had k values of 0.05-0.10/year, moderate-growing species had 0.10-0.20/year, and fast-growing species had 0.20-0.50/year, according to Branstetter (1987). The value of k computed for Rita rita from the three rivers was in the range of 0.12-0.15/year indicating a modest growth rate. A high metabolic rate is indicated by a high k value, and such fish mature at a young age or a large size to their asymptotic length (Qasim, 1973). Because there were fewer large specimens in this study, the L∞ value of Rita rita was larger than the maximum observed length. The growth coefficient (k) is a valuable metric for measuring fish vulnerability to depletion as well as comparing life history methods (Musick, 1999). The absence of very young or very old individuals has a significant impact on growth model estimations (Ma et al., 2010). The computed value of L∞ in Rita rita was 90.19, 91.19 and 89.63 for the River Ganga, River Yamuna and River Ramganga, respectively. Sabah and Khan (2014) found a higher value of L∞ than the recorded maximum length, which was likely due to the small number of large specimens (Karlou-Riga & Sinis, 1997). In the current study, the estimate of t0 ranged from -0.96 to -1.18, which is an excellent predictor of the accuracy of the estimated ages (Kerstan, 1985). Sparre and Venema (1998) reported that growth parameters varied from species to species, even in the same species and also from different stocks due to differing environmental conditions such as temperature, water quality, food availability, etc. Low temperatures can reduce the growth rate of ectotherms (Angilletta Jr et al., 2004).
The age group 2, 3, and 4 were dominant age classes, and these age classes were exploited more by the fishermen. After these age groups, exploitation decreased with the increase in the age of fish. Net size and mesh size is an important factor for the capture of fish. Generally, fishermen use nets having small to moderate mesh size. This form of fishing has an impact on juvenile and brooder stocks. The number of juvenile and brooder samples should be greater than the number of middle age class samples for healthy stock and heavy recruitment (Dwivedi et al., 2017). Since the length at first maturity of Rita rita is reported to be 29.5 cm (Saxena, 1972), the fish population in the present study appears to be dominated by broodstocks. The status of the age structure is an important indicator of how fished populations are responding to fishing pressures and stressors (Sarkar et al., 2006). The findings of the present study may be utilized for devising scientific and efficient strategies for the management of Rita rita fish species inhabiting the rivers, Ganga, Yamuna, and Ramganga. To ensure the species' long-term viability, suitable management measures must be established.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio