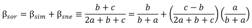Introduction
Fishes are the most biodiverse organisms among vertebrates, with approximately 36 018 currently recognized species, among which 18 185 are freshwater fishes (Fricke et al., 2022). Despite the relevance of this group in ecological, economic, and social terms (Hermoso et al., 2018), freshwater fishes face the highest number of threats and are less known than other vertebrates (Darwall et al., 2011; Miqueleiz et al., 2020). Freshwater ecosystems are among the most threatened ecosystems worldwide; they are disappearing faster than terrestrial ecosystems (Reid et al., 2019).
On the other hand, the Neotropical region is one of the regions with the highest fish diversity on Earth. Currently, nearly 6 200 freshwater fish species have been described (Albert et al., 2020), making this region an area with a unique biological heritage. The Magdalena basin, with 235 fish species, is in the Northwestern tropical region of South America (DoNascimiento et al., 2021). Although the diversity in the Magdalena basin is less than that of the Amazon and Orinoco basins, this basin is considered one of the regions on the planet with the highest percentage of endemism (68 %), with a total of 158 species (García-Alzate et al., 2020). In addition, fish species of the Magdalena basin provide multiple ecosystem services, such as food for people and another biota. Also, these species influence the local economy, culture, and recreation and contribute to ecosystem maintenance (Valderrama-Barco et al., 2020).
The composition and structure of the communities have been associated with the elevation gradients and changes in environmental factors along the cline. At a local scale, the pattern observed in the Magdalena basin is a decrease in species richness and an increase of endemism with the increase in elevation (Carvajal-Quintero et al., 2015; Herrera-Pérez et al., 2019) and the composition of fish communities have been the result of these same conditions (Albert et al., 2011). In mountain rivers, higher elevation areas have a high variation in riverbed slopes, higher water velocities, turbulence, and more oxygenated waters. In lower elevations, water systems are less turbulent and oxygenated (Jacobsen, 2008). Therefore, different aquatic environments within a fluvial network could play a crucial role in how communities structure through dispersion and environmental selection processes (Altermatt, 2013). Moreover, the Northwestern of the Andes is the output of a long geological history of isolation due to the differential uplift of physical barriers over time, in which individuals of each fish species survive new biotic and abiotic environmental conditions (Lévêque et al., 2008).
The loss of biodiversity in freshwater ecosystems reveals a rapid decrease in populations and a great risk of extinction in freshwater organisms (Reid et al., 2019). The Magdalena basin is no exception, as aquatic ecosystems are among the most affected by human activity in Colombia (Angarita et al., 2018; Jiménez-Segura et al., 2016; Rodríguez, 2015). Threats such as fisheries pressure and non-native fish species have already been reported (Hernández Barrero et al., 2021; Lasso et al., 2020). Cattle farms and agriculture on the floodplains have reduced the area of the floodplain lakes and their connection with the river. Water pollution due to poor sewage treatment in the cities, sediment retention, and hydrological change because reservoirs alter the river structure and dynamics (Angarita et al., 2020). The Magdalena River basin produces 60 % of the hydropower in Colombia; the dams block 50 % of the fluvial net in this basin, and the energy production changes the flood pulse (Angarita et al., 2018). As a result of the interaction of these threats, ~ 48 % of fish species are included within some of the IUCN threaten categories (Mojica et al., 2012; Tognelli et al., 2019), and artisanal fisheries landings have plummeted (Hernández Barrero et al., 2021).
Monitoring is a valuable strategy to detect changes in critical variables over time (Conly & Van Der Kamp, 2001; Roero et al., 2016); biota monitoring is one of the practical actions to detect any change in their composition, richness, and assemblage structure (Loures & Pompeu, 2018; Valencia-Rodríguez et al., 2022a) and let us look for their causes. Here, we explore the spatial relationship between fish assemblages and the different aquatic environments in the middle and lower Cauca River basin (the main tributary to the Magdalena River) prior to the construction of the Ituango Dam. For the analyses, we used the monitoring data conducted over nine years to explore the composition of the ichthyofauna in the Cauca River. This analysis focuses on species richness and changes in fish assemblage between aquatic environments. In this study, we seek to (i) strengthen the knowledge of how the fish assemblage is conformed in the middle and lower Cauca River basin, considering the local environmental context prior to the construction of the Ituango Dam and (ii) quantify β diversity and its two components (turnover and nestedness) amongst local fish communities. Answers to these questions make it possible to understand the fish community's structure before dam construction. The results will help measure the magnitude of change caused by the dam based on the long-term data collected. This not only allows for progress in the diversity inventories but also provides opportunities for conservation.
Materials and methods
Study area: The Cauca River is in the Northwestern tropical region of South America. It begins at 3 600 m above sea level (m.a.s.l.) in the Colombian mountains, between Cauca and Huila departments, running 1 350 km northward and flowing into the Magdalena River at 50 m.a.s.l. The Cauca River has an average flow rate of 1 800 m3.s-1, with temporary variations due to climatic changes, with two rainy seasons (May-June, October-November) and two dry seasons (January-March, July-September) within the annual cycle. Before flowing into the Magdalena River, the Cauca River basin drains through lands transformed by agriculture, cattle farming, informal mining, and the expansion of urban and suburban areas (Jiménez-Segura et al., 2016).
In 2009, the Ituango Hydroelectric project's construction license in the Cauca River's middle section was approved by the Environmental Authority in Colombia. This is the most significant power generation project underway in Colombia, as it will generate 2 400 MW of energy, supplying 17 % of the overall hydropower installed capacity in Colombia. This study covers the middle and lower Cauca River basin (Fig. 1; AT1); the basin limits were according to (Pérez-Valbuena et al., 2015). A total of 58 sites were analyzed; the field visits were conducted between February 2010 and November 2018, the period before the dam's operation. Throughout these years, each site was sampled four times per year, twice during the dry season and twice during the rainy season, except for the years 2010 and 2015, in which it was only possible to make two visits, one in the dry season and the other in the rainy season during the first half of the year.
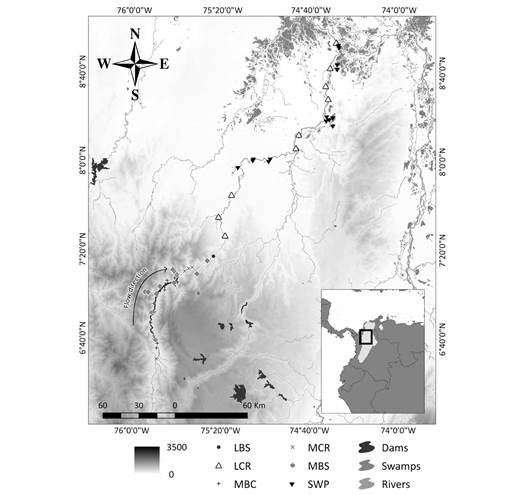
Fig. 1 Sampling design for ichthyofauna monitoring. MCR: middle Cauca River basin, MBC: middle basin creeks, MBS: streams flowing into the middle Cauca River basin, LCR: lower Cauca River basin, LBS: streams flowing into the lower Cauca River basin, and SWP: swamps.
Due to the selectivity of the sampling method for each species and the size of the fish, the same sampling effort was used for each site over time, depending on the environment sampled. In continuous flow channels (Cauca River, tributaries, and creeks), the sampling effort was 30 throws using three cast nets (different mesh sizes 1, 3, and 5 cm). In addition, to increase the probability of catching fish of different species and sizes, area sweeps were conducted using a portable electrofishing unit with one amp pulsed current (340 V, 1-2 A, dc) for 60 minutes in a 100 m-long transect over the main channel of the water body. In the swamps, the sampling effort involved two gill nets (each measuring 100 m long and 3 m high), one in the littoral zone and the other in the central zone. The time of exposure to gill nets was six hours (the maximum time allowed by the local human communities), from 17:00 to 23:00. Nets had ten different mesh sizes, ranging from 1 to 10 cm.
Fish data: Captured fish were anesthetized using eugenol solution to reduce stress during handling (Javahery et al., 2012). For complex taxonomic groups, a sample of 20 specimens was taken to the laboratory, which were included in the Ichthyology Collection at the Biology Institute of the Universidad de Antioquia in Medellín. The list of recorded species and the number of specimens collected are available in the supplementary files (AT2). The taxonomic classification is according to Fricke et al. (2022) and the most recent version of the Colombia checklist (DoNascimiento et al., 2021). The taxonomic determination followed several taxonomic studies for specific groups (Armbruster, 2005; Dahl, 1971; Harold & Vari, 1994; Hernández et al., 2015; Londoño-Burbano et al., 2011; Lujan et al., 2015; Ortega-Lara, 2012; Román-Valencia et al., 2013; Rosen & Bailey, 1963; Skelton, 2001).
Data analysis: To verify the quality of the information for each sampling site separately, a completeness analysis was performed (AT3). After verifying the quality of the information, the sampling sites were grouped into six aquatic environments. To select the samples sites according to the type of environment, a topological net proposed by López-Casas et al. (2018) was used for the Magdalena basin, which is based on a digital elevation model (SRTM, 90 m) and follows Strahler's river order classification (Strahler, 1957). Each sampling site was plotted on the topological network, and the associated information was extracted. Thus, creeks were assigned orders 1 and 2, tributary rivers were 3-5, and the Cauca River was assigned an order of 6, while swamps were assigned an order of 0 as they are lotic water bodies. After this classification, they were divided according to their geographical location in the middle or lower basin, as follows: MBC - middle basin creeks, MBS - streams flowing into the middle Cauca River basin, MCR - middle Cauca River basin, LBS - streams flowing into the lower Cauca River basin, LCR - lower Cauca River basin, and SWP - swamps (Fig. 1; AT1).
To estimate the number of species per aquatic environment (alpha diversity), we used the first three numbers of the Hill series (q0, q1, and q2) (Hill, 1973), following the method proposed by Jost (2006) and Chao et al., (2014), implemented in the iNEXT package (Hsieh et al., 2016). Where q0 is the effective richness, q1 is equivalent to the Shannon diversity exponent (i.e., common species), and q2 is equivalent to the inverse of the Simpson index (i.e., dominant species) (Jost, 2006). Estimated richness about observed richness allows for the estimation of the proportion of species recorded by the sampling, which varies between zero and one, where values near one suggest good representativeness in the number of species analyzed for the estimation diversity (Hsieh et al., 2016). We understand that each sampling method entails its own bias. We assume such bias occurs in the same way throughout all environments since all methods were used equally depending on the sampled environment. Thus, we used these estimations for relative comparisons among environments.
The difference in richness among aquatic environments was evaluated using the Kruskal-Wallis test; when a significant difference was detected (level of significance α = 0.05), post hoc comparisons were conducted in pairs, using the Wilcoxson sum rank test, with adjusted P value according to Holm's procedure (Holm, 1979). Holm's procedure is considered a more powerful correction (in other words, it is more likely to detect an effect if there is one) than Bonferroni's for multiple comparisons, providing improved protection against a Type 1 error (Wright, 1992).
To explore the differences in fish assemblages, we used multivariate, non-parametric methods. Resemblance matrices were generated using the Jaccard similarity index (presence/absence) that were compiled into a matrix of distances of fish species. Differences in the spatial distribution of similarities among environments were assessed with a permutational multivariate ANOVA (PERMANOVA) using the adonis function in the vegan package (Oksanen et al., 2015). To evaluate the differences between the assemblies of fish, we calculated the p-values corrected by Bonferroni with the pairwise.adonis function in the pairwise Adonis package in R (Martinez Arbizu, 2020). The results were displayed on a non-metric multidimensional scale graph (nMDS), considering the stress of 0.20 as an acceptable goodness of fit (Vinet & Zhedanov, 2010). Similarity-profile analysis was performed, and the results were overlaid on the nMDS plot to demonstrate the structure among samples.
To evaluate fish beta (β) diversity in the middle and lower Cauca River basin and determine how much spatial variation was due to richness differences and how much was due to species replacement, we calculated the Sørensen dissimilarity index (βsor) and its components: turnover (βsim) and nestedness (βnes; Equation 1) using the method proposed by Baselga and Araújo (2010). This index is based on presence-absence matrices and determines which of the components (βsim or βnes) underlies variations in β diversity through the following equation:
Where βsor is the Sørensen dissimilarity and is made up of the Simpson similarity (βsim), which consists of the substitution of species in one site for different species in another site (species replacement), describing a spatial turnover that is not influenced by differences in the species richness of each community, and βnes, which is the nestedness that occurs when sites with less species richness are a subgroup of the species at the sites with higher species richness (Baselga et al., 2022; Leprieur et al., 2011). All statistical analyses and graphs were performed in software R (R version 3.6.3) (R Core Team, 2020).
Results
The sampling integrity and representativeness values were similar among environments and exceeded 99 % (Table 1). The richness and the effective number of species of order q= 0 was greater in swamps, lower Cauca River basin, and middle Cauca River basin, and lower in tributaries flowing into the lower Cauca River basin (Table 1). The effective numbers of common species (order, q = 1) and dominant species (order, q= 2) were similar in middle basin creeks, streams flowing into the middle Cauca River basin, and lower Cauca River basin. From 2010 to 2018, a total of 109 893 fish specimens were collected in the 58 sampling sites, representing 11 orders, 33 families, and 114 species. The region with the most significant number of samples was swamping (77 032), lower Cauca River basin (16 029), and middle Cauca River basin (8 973) (Table 1). The Siluriformes (43 %) and Characiformes (38 %) orders represented 81 % of the total species sample, and Gymnotiformes and Cyprinodontiformes represented 7 and 8 %, respectively. Characidae was the family with the most species (18 %), followed by Loricariidae (16 %).
Table 1 Fish diversity indicators according to sampling sites.
| Indicators | MCR | MBS | MBC | LCR | LBS | SWP |
| Specimens | 8 973 | 2 658 | 4 558 | 16 029 | 643 | 77 032 |
| Species (S) | 79 | 58 | 63 | 81 | 30 | 92 |
| Extrapolated S | 90.1 | 68.1 | 68.8 | 93.5 | 34.9 | 96.2 |
| Common S | 11.9 | 20.4 | 20.1 | 19.2 | 9.4 | 16.9 |
| Dominant S | 4.9 | 10.8 | 11.9 | 11.7 | 6.4 | 8.9 |
| Unique S | 3 | 2 | 0 | 1 | 1 | 7 |
| Sample coverage | 1 | 0.99 | 1 | 1 | 0.99 | 1 |
*MCR: middle Cauca River basin, MBC: middle basin creeks, MBS: streams flowing into the middle Cauca River basin, LCR: lower Cauca River basin, LBS: streams flowing into the lower Cauca River basin, and SWP: swamps.
The most abundant species was Cyphocharax magdalenae (N= 21 465; 20 %), followed by Triportheus magdalenae (N= 8 684; 8 %) and Astyanax magdalenae (N= 8 032; 7 %). The least abundant species and those for which only one capture was recorded were Apteronotus magdalenensis, Apteronotus rostratus, Cetopsorhamdia boquillae, Characidium phoxocephalum, Cordylancistrus pijao, Dupouyichthys sapito, Lasiancistrus caucanus, Oreochromis mossambicus, and Saccodon dariensis (AT2).
The distribution of total richness of the species shows that the number of species was different amongst the environments (X2 = 38.4, P < 0.001; Fig. 2), where the swamps present the greatest number of species (SWP), with an average of 58.6 ± 5.5 of the total species in the study area, followed by the lower and middle Cauca River basins (LCR and MCR) with an average of 47 ± 7.6, and 35 ± 7 of species detected respectively. On the other hand, we identified that the aquatic environments (MBC and MBS) show similarities in the species richness values, with approximately 25 ± 5.7 of the total species. Regarding streams flowing into the lower Cauca River (LBS), we observed the lowest richness values (15.2 ± 6, mean ± SD). Thus, species richness is higher in the aquatic environments in the lower basin and decreases as the elevation increases towards the middle basin (Fig. 2).
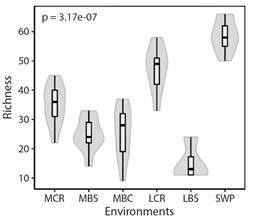
Fig. 2 Species richness according to environment. The shape of the violin diagram shows the data distribution, where the widest sections represent a greater number of species and narrower sections indicate a smaller number of species observed. P value from the Kruskal-Wallis test is shown for richness amongst environments. Environments were identified from the middle basin toward the lower basin, as follows: (MCR) middle Cauca River basin, (MBC) middle basin creeks, (MBS) streams flowing into the middle Cauca River basin, (LCR) lower Cauca River basin, (LBS) streams flowing into the lower Cauca River basin, and (SWP) swamps.
The nMDS and PERMANOVA analyses showed the spatial structure of the assemblage among the different aquatic environments. Fish assemblages in the swamp (SWP) and the main river channel of the Cauca River are discrete units (Fig. 3); the last one has differences in its middle and lower sections. In addition, fish assemblage in rivers and creeks flowing to the main river channel of the Cauca River could be considered a single environment unit (namely, tributaries) for fish (Fig. 3).
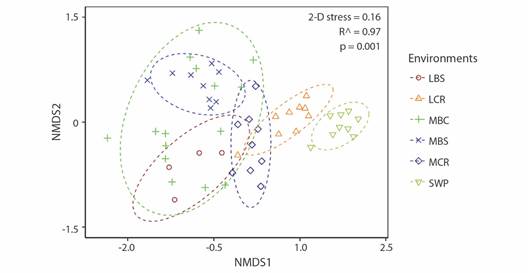
Fig. 3 Ordering of fish assemblages into aquatic environments using non-metric multidimensional scaling (nMDS), using Jaccard's similarity index. Environments are represented using symbols, and assemblage groups are represented with lines (70 % similarity). (MCR) middle Cauca River basin, (MBC) middle basin creeks, (MBS) streams flowing into the middle Cauca River basin, (LCR) lower Cauca River basin, (LBS) streams flowing into the lower Cauca River basin, and (SWP) swamps.
For average β diversity, we obtained a value for βsor of 0.94, a value for βsne of 0.06, and the β diversity component due to replacement (βsim) was 0.88 (AF1). Our results suggest that there is a high variation component in the study area due to species replacement (βsim) between the aquatic environments. In other words, the replacement of species is the component that most contributes to the general beta diversity of the area (AF1). The low percentage of β diversity that was not originated by differences in species composition is related to nestedness.
Discussion
The high percentage of endemic fish in the Magdalena basin is greater than in other mountainous places in the world (García-Alzate et al., 2020) due to the particular evolutionary processes in neotropical montane fish (Oberdorff et al., 2019; Schaefer & Arroyave, 2010). We detected that the number of species is lower in creek environments and is higher in the Cauca River surroundings. However, the number of species is higher in the lower part of the Cauca River based on the ordinary succession of fish species in the elevation gradient in the Andes (Lujan et al., 2013). The increase in ecological diversity, environmental stability, and the trophic resources accumulated and dragged downstream stand out as causal agents of the species increase, as already observed in previous studies (Benejam et al., 2018; Carvajal-Quintero et al., 2015; Herrera-Pérez et al., 2019). Thus, elevation is a determining factor in fish richness in the Magdalena basin (Herrera-Pérez et al., 2019). On the other hand, the variability of water conditions at the sampling sites is associated with seasonal precipitation changes, soil types, and forest cover at the riverbanks, among others (Rodrigues-Filho et al., 2018), but these topics were not the focus of these analyses.
The distribution of fish richness in the middle and lower Cauca River basins is similar to the general tendency of richness reported for other tributaries of the Magdalena River basin (Jiménez-Segura et al., 2016). For the study area, 49 % of the total richness of known species for the Magdalena-Cauca hydrographic system was recorded (DoNascimiento et al., 2021). The greatest number of captured species were recorded in the swamp and Cauca River main course environments, where the most frequent species included C. magdalenae, A. magdalenae, T. magdalenae, and Roeboides dayi, which are mostly potamodromous species widely distributed along the Andean region and are important for subsistence fishing and economy of the fishing population (Moreno-Arias et al., 2021). On the other hand, the dominant species in the creek and Cauca River tributary environments were Brycon henni, Argopleura magdalenensis, Poecilia caucana, and Creagrutus brevipinnis; all species are native to the Magdalena basin, and with ecological importance, in the environments, they inhabit (Valencia-Rodríguez et al., 2021). For example, some species contribute to the decomposition and recycling of nutrients. While others, being great predators, help maintain the balance of ecosystems by acting as population regulators (Botero-Botero & Ramírez-Castro, 2011).
The sampling effort indicates that the number of species obtained for most environments is representative, except for the Cauca River tributaries in the lower part of the basin, indicating that despite our efforts, we could not generate a representative sample in this environment; thus, any conclusion regarding its composition and richness must be taken with caution. The low number of individuals captured in this aquatic environment may be caused by their adverse conditions, such as strong currents with frequent removal of the biota caused by seasonal landslides, which limit the colonization of generalist, widely distributed species, and also the fishing method can be affected by the capture method, which is particular and selective for each aquatic environment (i.e., fish species and fish sizes). Therefore, conclusions may not be focused on if there were differences between the aquatic environments, but they may be on fish assemblage particularities. Hence, we emphasize the need to continue studying this area with efforts focused on the river and creek environments to strengthen the characterization of fish diversity in the Cauca River basin. Our results show relative particularities in the species diversity among environments. Most environments present unique species, among which the swamp sites contained the most significant number of such species (seven), while in the creeks of the middle basin, there were no unique species.
The knowledge about species distribution and their environmental requirements is not advancing as quickly as the transformations occurring in fluvial networks. Also, the modifications caused in physical environments can have repercussions on species distribution and abundance, both in plants and animals (Wetmore et al., 1990). The selection of an environment results from the decisions an individual makes to maximize their fitness (Koster et al., 2020). Usually, a high environment heterogeneity allows for the co-occurrence of multiple strategies and therefore hosts a higher number of species. In sum, species richness is greater as environment complexity increases in depth, water speed, and substratum conditions (Rodrigues-Filho et al., 2018). Thus, a connected fluvial network plays an essential role in the composition of these assemblages (Tonkin et al., 2018; Valencia-Rodríguez et al., 2022b). With this in mind, this study provides an understanding of how fish communities are structured in the middle and lower Cauca River basin, considering the natural conditions of the river. Additionally, our results show that the swamp environment offers different habitats for fish species and that these species' populations respond to this spatial heterogeneity. The opposite occurs in creek and tributary environments, as they are redundant among each other and are nested.
After nine years of observation, and under our classification of environment units, we observed that the distribution of the assemblages is particular to each aquatic environment, as the main discrepancies were observed in the elevation gradient. The lacustrine conditions of swamps and environmental gradients, such as elevation, favor fish distribution, grouping them in various assemblages throughout the basin. For example, larger species, such as potamodromous fish, are found in the lower part of the basin (López-Casas et al., 2016; Moreno-Arias et al., 2021). Whereas, at higher elevations, we find smaller species such as Astroblepus, Trichomycterus, Poecilia, and Hemibrycon, among others. Thus, the results of our study support the hypothesis that environmental gradients affect fish community composition (Herrera-Pérez et al., 2019).
The β diversity values suggest a high component due to species replacement, which indicates that within the system sampled, there is a high proportion of endemism or multiple sites with unique species (Baselga, 2010). This β diversity pattern may probably be modified over time due to the new conditions generated by the new surrounding (Ituango Dam). Such conditions lead to the homogenization of the environment due to the decrease in native species and the establishment of new non-native species (Agostinho et al., 2008; Poff et al., 2007; Rolls et al., 2021); this has also been described in local systems of the same basin (Valencia-Rodríguez et al., 2022a).
We need to keep in mind that the changes in physic-chemical conditions and even in processes, such as the change in the use of the soil in the hydrographic basin, can be relevant over the period of time the data came from (Jiménez-Segura et al., 2016). An analysis of how the environment and the physic-chemical conditions of the shallow waters of the Cauca River have changed and whether these changes could serve as plausible alternatives or synergic mechanisms that lead to a response in the fish community is beyond the scope of this study. However, it would be a good topic for future research. Our study will serve as a baseline to verify, over time, whether the construction of the Ituango Dam is associated with essential changes in the structure of fish communities. However, we cannot provide a complete mechanistic assessment of the processes that generate these effects without considering the physic-chemical changes in the quality of the water.
Lastly, our study provides knowledge on how the fish community was structured prior to the construction of the Ituango Dam, which will stimulate changes in the Cauca River structure, both up and downstream from the dam, as well as in the adjacent lotic environments that will not be affected by the transition from lotic to lentic systems. Therefore, it is necessary to continue observing species composition in this part of the basin, making it possible to detect eventual changes in the structure of the fish community. This is also necessary because some of the species captured during these nine years of observation have not yet been taxonomically classified, and they may represent species that are potentially undescribed or that have not previously been recorded. This would modify the inferences regarding endemism in this region and reinforce the need for further research on aquatic environments in this basin. Although it is likely a future scenario where no hydrographic basins in Colombia will remain intact due to various factors that exert pressure on aquatic ecosystems, we recognize that the middle and lower Cauca River basin preserves a great variety of species-rich environments. Therefore, knowing which groups will be the most affected along the lotic sections near the dam will help determine conservation strategies.
Ethical statement: This study was carried out with recommendations and approval of the Ethics Committee for Animal Experimentation from the Universidad de Antioquia (CEEA). Protocol was reviewed and approved on November 14 of 2017 by CEEA and the investigation was approved on December 7 of 2017. The specimens were collected under collection license number 0524 of May 27, 2014.












 uBio
uBio