Introduction
Clown anemonefish (Amphiprion ocellaris) belongs to the subfamily Amphiprioninae (family Pomacentridae) with 28 species and are the most popular and commercial exploited species of fishes in the marine aquarium ornamental trade (Khoo et al., 2018; Khoo et al., 2019). These species are considered as a model for scientific research, especially in nutritional studies, initial egg ontogeny and the quality of larvae (Delbare et al., 1995). Several studies in A. ocellaris report the reproductive biology, early development, including embryology and larval stage, osteological development of the vertebral column and caudal complex, growth pattern and natural diet (Khoo et al., 2018; Khoo et al., 2019; Madhu et al., 2012; Rodríguez-Ibarra et al., 2017; Yasir & Qin, 2007).
Even embryo development organogenesis information is available in the species. Nevertheless, larval ontogeny includes the transition between endogenous to exogenous energetic reserves, a period considered as a bottleneck in ornamental aquaculture species (Olivotto et al., 2011), where several morpho-physiological processes take place, including digestive enzyme development (Chen et al., 2019; Wilson & Castro, 2010; Zambonino-Infante & Cahu, 2001). In this sense, nutrient availability for metabolic functions is directly influenced by the enzyme digestion process, which depends mainly on the type of digestive enzymes (Nazemroaya et al., 2015). The development of specific feeds for larvae requires a detailed knowledge of their digestive physiology because of many marine fish larvae show null or a rudimentary digestive system at hatching and during digestive development, the digestion, absorption, and nutritional requirements are in constant change (Rønnestad et al., 2013; Shin et al., 2022). In addition, the digestive system development varies in time, depending on the ontogeny type (direct, transitional, or indirect) of each species (Balon, 1986; Balon, 1990; Peñaz, 2001), where many marine fish larvae lack a functional stomach and they depend on pancreatic proteases for protein digestion (Rønnestad et al., 2013). Thus, an understanding of the changes in the ability to digest and absorb during each larval stage is needed to improve productivity.
Yúfera et al. (2018) proposed the classification of enzymes during ontogeny by the pattern of appearance, using normalized data on degree days and the maximum activity detected in each enzyme type. Therefore, enzymes could be classified as precocious, medium, or late enzyme development. According to previous studies in A. ocellaris, at hatching, larvae possess an advance development on their digestive system (Madhu et al., 2012; Yasir & Qin, 2007). Nevertheless, by a short period time, larvae show embryo vestiges such as yolk-sac, and is still developing several systems, for this reason A. ocellaris, as well to other Amphiprion species, shows a transitional early ontogeny development, characterized by a long embryonic period before hatching and short larvae period before juvenile transforming.
Therefore, larvae culture requires the characterization of digestive system changes during larvae development to understand specific species adaptations and generate specific feeding protocols and development of specific feeds. Many studies are focus to characterize the development of the digestive system during the early ontogeny, including golden pompano (Trachinotus ovatus) (Ma et al., 2014), sobaity sea bream (Sparidentex hasta) (Nazemroaya et al., 2015), black tetra (Gymnocorymbus ternetzi) (Lipscomb et al., 2020), red sea bream (Pagrus major) (Khoa et al., 2019), japanese flounder (Paralichthys olivaceus) (Khoa et al., 2021), japanese eel (Anguilla japonica) (Shin et al., 2022). However, to our knowledge, only one report on digestive enzymes in A. ocellaris exists, showing the gastric emptying and pepsin activity on juvenile stage (Khoo et al., 2019).
Despite the economic importance of clown anemonefish commercialization, information on the enzymatic profile and digestive capacity of the specie is scarce. In addition, the great diversity, and variants of the development between species made necessary to characterize each species. Hence, the aim of the present study was to characterize the ontogeny of digestive proteases and lipase in clown anemonefish larvae (A. ocellaris).
Materials and methods
Larvae rearing: Fertilized eggs were obtained from an A. ocellaris couple spawned in Centro de Investigación en Alimentación y Desarrollo A.C. (CIAD), Mazatlán. Eggs were transferred to three 300 l water volume tanks for presumptive density of 1.5 larvae/l. Temperature was maintained at 26 ± 1.0 °C, salinity of 35 ± 1.0 g/l and a dissolved oxygen concentration of 6.2 mg/l. Tanks had constant aeration (1-2 l/min) with 30 to 40% water exchanges every third day by tank siphoning until day 13. From day 14, continuous water flow was adjusted to start with two total changes per day, reaching four total water changes at the end of the experiment. The larvae total length (TL) was measured (± 0.01 mm) with a binocular microscope and larvae growth was determined as the total growth rate (TGR) as mm/day: TGR = (final TL - initial TL) /days.
The feeding protocol is shown in Table 1. Briefly, larvae were reared using the green water technique from the 1st to 6th day after hatching (DAH) using microalgae Nannochloropsis oculata, from the 2nd to 9th DAH rotifers of the species Brachionus rotundiformis were added. From the 7th to 16th DAH, nauplii of Artemia sp. were added, while from the 14th to 38th DAH, a formulated diet (Skretting) was used. During early development, 10 samples per tank of egg just before hatching (day 0) and larvae at 1, 3, 5, 8, 14, 20, 28 and 38 DAH were taken in 1.5 ml Eppendorf tubes and frozen at -80 °C for two days, lyophilized and stored in dry conditions at -20 °C until analysis. For comparisons between closely related species, thermal age units (cumulative degree-DAH, CTU) were used.
Table 1 Feeding schedule and food types during clown anemonefish (Amphiprion ocellaris) larvae rearing.
| Food item | Type | Amount | Period (DAH) |
| Microalgae | Nannochloropsis oculata | 500 000 cel/ml | 1-6 |
| Rotifer | Brachionus rotundiformis | 10 rotifer/ml | 2-6 |
| Rotifer | Brachionus rotundiformis | 5 rotifer/ml | 7-9 |
| Artemia | Artemia sp. nauplii | 6 nauplii/ml | 7-13 |
| Artemia | Artemia sp. nauplii | 3 nauplii/ml | 14-16 |
| Dry microdiet | Formulated diet (Scretting) | At libitum | 14-38 |
Preparation of enzymatic extracts: Three larvae pool (10 larvae each pool) were homogenized separately in a 1:100 (weight: saline solution 0.9 % NaCl) ratio using an Ultra Turrax (IKA T18 basic, Wilmington, USA). The homogenates were centrifuged (14 000 g for 15 min at 4 °C) and the supernatants were recovered and stored (-80 °C) until analysis. The Bradford (1976) technique was used to determine soluble protein concentration in the larvae enzymatic extracts at each stage.
Digestive enzyme activity analysis: Acid proteases activity was determined using bovine hemoglobin 1 % as substrate in 100 mmol/l glycine-HCl buffer at pH = 2 (Anson, 1938). Alkaline proteases activity was determined using casein 1 % as substrate in 100 mmol/l Tris-HCl + 10 mmol/l CaCl2 buffer at pH = 9 (Walter, 1984). The trypsin activity was determined using Na-Benzoyl-DL-arginine-4-nitroanilide hydrochloride (BAPNA) 1 mmol/l as substrate in 100 mmol/l Tris-HCl + 10 mmol/l CaCl2 buffer at pH = 8 (Erlanger et al., 1961). Chymotrypsin activity was quantified using SAAPNA (N-succinyl-ala-ala-pro-phe p-nitroanilide) 0.1 mmol/l as substrate in 100 mmol/l Tris-HCl solution + 10 mmol/l CaCl2 buffer at pH = 7.8 (Del-Mar et al., 1979). The leucine-aminopeptidase activity was measured using leucine p-nitroanilide 1 mmol/l as substrate in 50 mmol/l monobasic sodium phosphate buffer at pH = 7.2 (Maroux et al., 1973).
The lipase activity was measured using 4-Nitrophenyl acetate 200 mmol/l as substrate in buffer 50 mmol/l Tris-HCl at pH = 7.2 with sodium taurocholate 100 mmol/l. Activity revelation was performed using Fast blue solution (100 mmol/l) and was clarify by adding ethanol: ethyl acetate solution (1:1 v/v) (Versaw et al., 1989).
All enzymatic activities were performed at 37 °C by triplicated and products were quantified in a Genesys 10S UV-Vis spectrophotometer (ThermoScientific, Waltham, MA, USA). The tyrosine released from hemoglobin and casein hydrolysis was determined at 280 nm, the amount of p-nitroanilide released from BAPNA, SAAPNA and L-leucine-p-nitroanilide hydrolysis was determined at 410 nm and the amount of p-nitrophenol released from 4-Nitrophenyl acetate hydrolysis was determined at 405 nm.
Total activity (Units/ml) = (Dabs*reaction final volume (ml)) / (MEC*time (min)*extract volume (ml)) and Specific activity (Units/mg prot) = Total activity / soluble protein (mg/ml), where Dabs represent the increase in absorbance, and MEC represents the molar extinction coefficient of tyrosine, p-nitroaniline and p-nitrophenol (0.005, 0.008 and 0.02 ml/µM/cm, respectively).
Native and SDS-PAGE zymogram: Mini-PROTEAN 3 Cell (Bio-Rad) with four vertical plate gels (8 × 10 × 0.075 cm) with 10 ml sample capacity per plate was used for the electrophoretic analyses.
Acid proteases electrophoresis was run under non-denaturing native conditions (Native-PAGE) with continuous polyacrylamide (PAA) (10 %) in Tris (25 mmol/l) and glycine buffers (192 mmol/l, pH = 8.3, 80 volts) according to Davis (1964). Alkaline proteases electrophoresis was run under denaturalizing conditions (SDS-PAGE), with PAA stacking a gel (4 %) and PPA resolving gel (10 %), adding SDS (0.1 %) in Tris buffer (25 mmol/l) and glycine (192 mmol/l, pH = 8.3, 100 volts), according to Laemmli (1970) and adapted by García-Carreño et al. (1993).
Native-PAGE electrophoresis gels were revealed for proteases isoforms according to the procedure of Díaz-López et al. (1998). The removed gels were soaked during 15 min in 100 mmol/l Tris-HCl in pH = 2.0 to activate enzymes. Then, gels were submerged during 60 min at 25 °C in 0.25 % hemoglobin solution in Glycine-HCl buffer (100 mmol/l) at pH = 2.0. The gels were washed with distilled water and fixed in 12 % trichloroacetic acid (TCA) solution for 15 min. SDS-PAGE electrophoresis gels were washed and incubated during 60 min at 4 °C in a 0.5 % casein solution (Tris-HCl 100 mmol/l buffer, pH = 9). Then, gels were incubated for 60 min at 37 °C in the same solution, and then washed and fixed in TCA as previously described. For Native and SDS-PAGE, gels were stained using a 0.1 % Coomassie brilliant blue R-250 solution, while distaining was carried out in a 35:10:55 solution of methanol-acetic acid-water (Weber & Osborn, 1969). Clear zones revealed the activity of proteases, the bleaching process was maintained until well-defined zones were obtained (after 2-4 h).
The protein leader (Termo Scientific; Cat# 26614) comprised of 14 recombinant proteins (weighting from 10 kDa to 200 kDa) was used as molecular markers and 5 µl per well was applied to each SDS-PAGE electrophoresis. Molecular weight (MW) of each band was calculated using a linearly adjusted relationship between the Rf (migration distance of the protein/ migration distance of the dye front) and log10 of the MW of each recombinant protein.
Statistical analysis: The specific activity (U/mg protein) was analyzed for normality (KS) and homoscedasticity (Levene) postulates and one-way analysis of variance (ANOVA) was applied to determine if differences exist between age and enzyme activities. When differences exist between treatments, a posteriori Tukey test was used. All tests were carried out using a level of significance of 0.05. The Sigma Plot (Systat Software inc., 2009) was used to perform the statistical analyzes.
Results
Growth performance: At hatching, A. ocellatus larvae showed a total length of 4.5 ± 0.13 mm, increasing to 14.73 ± 1.01 mm at 38 DAH, displaying a TGR value of 0.2 mm day-1. Growth of A. ocellatus larvae fit to an exponential model (r2 = 0.9506) (Fig. 1).
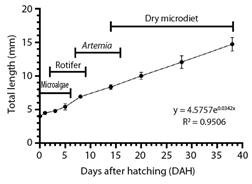
Fig. 1 Larvae growth curve in total length (mean ± SD) of clown anemonefish (A. ocellaris) from hatching to 38 DHA.
Enzyme activity: For all enzymes, activity was detected from the moment of hatching (1 DAH), with values of 0.150, 0.416, 0.167, 41.65, 0.812 and 10.01 U/mg protein for acid proteases, alkaline proteases, trypsin, chymotrypsin, LAP, and lipase respectively. The specific activity (U/mg protein) and relative activity (%) against degree days of acid proteases shows two peaks at day five (130 CTU) and day 28 (728 CTU), decreasing to day 38 (988 CTU) (P ≤ 0.05), while total alkaline proteases show a peak at day 8 (208 CTU) and then decreased from day 14 (364 CTU) to day 38 (988 CTU) (P ≤ 0.05) (Fig. 2A, Fig. 2B). The trypsin, chymotrypsin and leucin aminopeptidase activity shows the same pattern in specific activity (U/mg protein) and relative activity (%) against degree days, it increased from hatching with a peak at day 8 (208 CTU) and then decreased from day 14 (364 CTU) to day 38 (988 CTU) (P ≤ 0.05) (Fig. 2C, Fig. 2D). The lipase specific activity (U/mg protein) and relative activity (%) against degree days showed two higher peaks at day 8 (208 CTU) and day 28 (728 CTU), decreasing at day 38 (988 CTU) (P ≤ 0.05) (Fig. 2E, Fig. 2F).

Fig. 2 Specific enzyme activity (U mg protein-1) and relative activity (%) in larvae development of clown anemonefish (A. ocellaris) (mean ± SD). Statistical differences (P < 0.05) against stages DAH are indicated by superscript letters. A. Specific activity of acid and alkaline proteases; B. Relative activity of acid and alkaline proteases against degree days; C. Specific activity of trypsin, chymotrypsin, and leucine-aminopeptidase; D. Relative activity of trypsin, chymotrypsin, and leucine-aminopeptidases against degree days; E. Specific activity of lipase; F. Relative activity of lipase against degree days.
Electrophoretic analyzes: Acid zymogram shows a single band with acid activity during all ontogeny, which appears at the 8th DAH and increases its activity until the 38th DAH (Fig. 3A). Alkaline zymogram shows a total of eight bands (154.2, 128.1, 104.0, 59.8, 53.5, 41.9, 36.5 and 25.1 KDa) (Fig. 3B). At 0 DAH, no bands were detected. On the 1st DAH (26 CTU) the heaviest seven bands were revealed, from the 3rd (78 CTU) to 8th DAH (208 CTU), eight bands were revealed. At the14th DAH (364 CTU), three bands were revealed (41.9, 36.5 and 25.1 KDa), at the 20th DAH (520 CTU) four bands were revealed (154.2, 128.1, 41.9 and 25.1 KDa), while from the 28th (728 CTU) to 38th DAH (988 CTU) two bands were revealed (41.9 and 25.1 KDa).

Fig. 3 View of the electrophoretic gels. A. Native-PAGE; B. SDS-PAGE electrophoresis analysis of clown anemonefish (A. ocellaris) during larvae ontogeny from hatching to 38 days after hatching (DAH). Molecular Marker (MW) protein leader (Termo Scientific; Cat# 26614) comprised by 14 recombinant protein weighting from 10 kDa to 200 kDa.
Discussion
The sequence apparition of most developmental events in fishes are mostly stable, only varying in rate and duration of yolk sac depletion, that depends on the size of the yolk-sac (Peñaz, 2001). Newly hatched fish larvae initially depend on endogenous reserves for survival, but they must switch to exogenous feeding before their yolk reserve depletes (Chen et al., 2019); however, this switch is variable in time, depending on the development stage at hatching time that defines the ontogeny type of each species (Balon, 1986; Balon, 1990; Peñaz, 2001). In this sense, A. ocellaris possess an advanced development of the digestive system at hatching (Madhu et al., 2012; Yasir & Qin, 2007). Nevertheless, by a short period time, larvae show embryo vestiges such as yolk-sac, and is still developing several systems, for this reason A. ocellaris, as well as to other Amphiprion species, shows a transitional early ontogeny development, characterized by a long embryonic period before hatching and short larvae period before metamorphosing into a juvenile.
The enzymatic characterization in the present study was performed from the embryonic period (before hatching), the eleutheroembryo period, from hatching and the last day until yolk sac is digested (from the 1st to 3rd DAH) and larvae period, with two phases: Protopterygio phase (encompasses the interval between transition to exogenous feeding; from the 4th to 7th DAH) and Pterygiolarvae phase (the jaws are well developed into functional structures); and the juvenile period represents the time when transition to definitive organs is completed between the 15th to 17th DAH (Madhu et al. 2012; Yasir & Qin, 2007).
Reports in A. ocellaris shows that newly hatched larvae show the lower jaw formed, but the mouth is not yet open, however in a few hours, the mouth is visible with 170 to 210 µm, able to detect and ingest exogenous food on the first day after hatching, related to well-developed sensory and olfactory systems (Madhu et al., 2012; Yasir & Qin, 2007). Studies in fire clownfish (A. melanipus) shows that the gut is already looped and differentiated (foregut, midgut, and hindgut) with large intestinal lumen and visible food inside the lumen at hatching, present compact and granular liver, with hepatocytes lacked vacuoles (Green & McCormick, 2001). In tomato clownfish (A. frenatus) larvae at 24 h after hatching show an evident alimentary tract with gut and liver differentiated. At the 2nd DAH, a well-developed alimentary tract is evident, with a distinct stomach, midgut and hindgut, liver, and pancreas (Putra et al., 2012). In orange clownfish (A. percula), the alimentary tract, liver and pancreas are present at hatching, showing absorptive and digestive capabilities, where larvae start exogenous feeding immediately (Gordon & Hetch, 2002; Önal et al., 2008). Red saddleback anemonefish (A. ephippium) hatch at day 6 post fertilization and at that moment show open mouth and start feeding by itself and at the 2nd DAH, larvae show a well-developed digestive system (Rohini et al., 2018). In this sense, the present study shows that A. oceallaris presents acid proteases, alkaline proteases, cytosolic proteases, and lipases at hatching, which corroborates the existence of a functional stomach, pancreas, and intestine with the capacity to acquire exogenous reserves from food digestion in combination with the use of endogenous reserves from the yolk sac.
In the present study, alkaline proteases and lipase show activity from hatching and increase from first day until the 8th DAH. Complementary, seven active digestive alkaline proteases were detected in the zymogram (154.2, 128.1, 104.0, 59.8, 35.5, 41.9 and 36.5 KDa) from the 1st DAH, increasing one more active band (25.1 KDa) until the 8th DAH and decreasing in activity and digestive bands from that moment, showing only two alkaline proteases (41.9 and 25.1 KDa) at the 38th DAH. In A. percula, acidophilic zymogen granules precursors of pancreatic enzymes are accumulated in exocrine pancreatic cells just before hatching (Önal et al., 2008; Zambonino-Infante & Cahu, 2001), indicating that digestive machinery is ready. Therefore, the increasing number of alkaline proteases until the 8th DAH is related to rapidly morphophysiological changes such as expansion of support organs (liver and pancreas), increase in mean gut epithelium cell height (increase with age and size), increase in surface area for enzyme secretion, digestion of nutrients and absorption, developed vacuoles in the intestine as functional areas of lipid and glycogen storage that are of the extracellular digestion of lipids, diffusion of fatty acids into enterocytes and re-synthesis of triglycerides in to the enterocytes (Green & McCormick, 2001; Gordon & Hetch, 2002; Zambonino-Infante & Cahu, 2001).
In gastric fishes, the development of a functional stomach with pepsin-like enzyme secretion is associated with the transition from larvae to juvenile, critical to evaluate the digestive capacity of fish larvae, especially when considering the weaning time (transition from live prey to formulated feeds) (Kolkovski, 2001; Salze et al., 2012; Zambonino-Infante & Cahu, 2007). Reports in A. ocellaris show that at the 7th DAH, gastric glands are established in the epithelium of the stomach, being capable of digesting formulated diets at 10 DAH (Gordon & Hetch, 2002). In the present study, A. ocellaris shows a peak of activity in acid proteases at 5th DAH, appearing one pepsin-like enzyme band (Rf= 0.72) on the acid zymogram at 8th DAH, showing an increase in activity and thickness of bands until 38th DAH, associated to a rapid stomach growth, secretions increase, as well as to functionality of time retention (Khoo et al., 2019; Salze, et al., 2012). Reports in A. percula shows that gastric glands are established in the epithelium between the 7th DAH (Gordon & Hetch, 2002) and 11th DAH (Önal et al., 2008). In A. melanopus, the stomach is visible as a slight enhancement of the esophagus at the 3rd DAH, with a sphincter in each end, and lined with cuboidal epithelium with many secretory cells (Green & McCormick, 2001). Therefore, the decrease in alkaline proteases activity and the increase in acid proteases activity showed between the 8th and 14th DAH, correspond to the turnover to a juvenile period in A. ocellaris. Laboratory management of A. ocellaris in CIAD-Mazatlán begins with a dry diet feeding at the 14th DAH, however, these results show that early weaning time can be achieved in the species, where reports in A. percula shows that can be weaned off from 7th DAH (Gordon et al., 2000).
A. ocellaris is considered as an omnivorous species because the stomach content, showing a variety of larvae and algae (Khoo et al., 2018), and possess a high support of protein digestive capacity by the stomach, showing a pepsin activity peak at 2 h after feeding with long time food retention (36 h), where the gastric secretions are regulated effectively with infrequent and irregular meals (Khoo et al., 2019). The switch of acid to alkaline proteases between the 8th and 14th DAH found in the present study, support that protein digestion in the species is mainly produced by the stomach, showing only two alkaline proteases at the 38th DAH. Even if A. ocellaris feeds on animal and vegetable protein in their natural environment, the diversity of digestive alkaline proteases at juvenile stage is reduce, therefore could be classified as an omnivorous species with carnivory tendency. However, more studies on digestive enzyme functional characterization, as well to digestibility of different protein and lipid sources by enzymatic battery of the species is required.
Roux et al. (2021) presented standard methods to maintain breeding pairs of A. ocellaris in captivity to establish regular good quality spawning, and protocols to ensure larval survival, with the objective to use the species as experimental marine model. In this study, a homemade feed was manufactured for breeders to ensure nutritional quality, however, larvae nutrition was established using green microalgae, rotifers and Artemia nauplii. Therefore, the proposed method can be improved by the understanding of the digestive capacity of the species, considering breeders and larvae to design specific diets for the different life stages of A. ocellaris.
According to Yúfera et al. (2018), A. ocellaris shows a precocious enzyme development on total alkaline proteases, trypsin, chymotrypsin, and LAP, peaking at 8th DAH (208 CTU), while pepsin and lipase could be classified as late enzyme development, peaking at 28th DAH (728 CTU). However, lipase showed 98 % of relative activity (%) at 8th DAH regarding to maximum activity, therefore showed two major peaks, and could be considered as precocious. Therefore, A. ocellaris is a precocious species in protein digestion mainly supporting alkaline digestion by pancreatic (trypsin, chymotrypsin, and lipase) and intestine (membranous and cytosolic enzymes) enzymes (Zambonino-Infante & Cahu, 2007), showing a switch to stomach digestion by pepsin in the last ontogenic days.
In conclusion, clown anemonefish (A. ocellaris) shows pancreatic and luminal active digestive enzymes at hatching, increasing in activity and enzyme number until the 8th DAH, time at which an enzymatic switch by the increasing activity of one acid protease and decrease of alkaline proteases in activity and number, however lipase increased its activity on juvenile stage. Therefore, the protein digestive capacity in A. ocellaris is mainly supported by the stomach, showing an enzymatic pattern of an omnivore fish with a tendency to carnivory.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.
All procedures of fish manipulation, including the euthanasia method by thermal shock followed the Mexican legislation according to Norma Oficial Mexicana NOM-062-ZOO-1999 from Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), the Mexican standards for good welfare practices of laboratory animals.












 uBio
uBio 


