Introduction
Scleractinian coral sexual reproduction involves the fertilization of eggs that develop into swimming larvae. Upon settlement, these larvae undergo a metamorphosis with polarity reversal to develop a sessile polyp, which reproduces asexually by budding to grow into a colony with the ability to mature gametes (Fadlallah, 1983; Harrison & Wallace, 1990; Richmond, 1990; Sammarco, 1994). The larvae possess lipid vacuoles, and these serve as energy repositories that contribute to their positive buoyancy (Vandermeulen, 1974); which contributes to the maintenance of the larvae in the plankton (up to 100 days) and allow a more strict selection of the substrate (Richmond, 1987; Wilson & Harrison, 1998) and disperse to new habitats and promotes gene flow (Ayre et al., 1997).
Overall, early development involves cell division that forms a blastula and a subsequent upper and lower ectoderm excision resulting in a second tissue layer, which develops as the endoderm (Babcock & Heyward, 1986; Ball et al., 2002; Hirose & Hidaka, 2006; Okubo et al., 2013). Cilia allow planulae to swim with an oral orientation (Ball et al., 2002; Gleason & Hofmann, 2011). The aboral pole acts as a sensor for recognizing suitable settling sites (Chia et al., 1984; Vandermeulen, 1974). Larval settlement is regulated synergistically by multiple tactile and chemical cues (Gleason & Hofmann, 2011; Ritson-Williams et al., 2009), allowing an adequate selection of substrate and survival advantage of these organisms (Kitamura et al., 2007; Müller & Leitz, 2002). Even though larvae have chemoreceptors, the chemotaxis from a distance and in highly dynamic environments is not strong enough to detect an appropriate substrate, but specific factors are likely to be present directly on its surface (Müller & Leitz, 2002). Therefore, specialized cells should be a key in the detection of both chemical and mechanical forces such as compression or tension (Katta et al., 2015), and cellular structures present in the ectoderm of the aboral zone have been associated with larval settlement (Chia & Koss, 1979; Martin, 1983; Vandermeulen, 1974).
The mode and reproduction patterns of scleractinian corals are both species-specific and spatiotemporally variable (Chávez-Romo et al., 2013; Santiago-Valentín et al., 2018). At the regional level, the Eastern Tropical Pacific (ETP) environmental conditions are considered limiting for the development of coral communities (Dana, 1975; Glynn et al., 1996; Richmond, 1990). Conditions such as the wide ranges of annual temperature fluctuations (18 to 32 °C), low pH (7.72 to 8.03) (Cupul-Cortés et al., 2018), high sedimentation rates (Glynn et al., 2017), seasonal upwellings (Portela et al., 2016) and internal waves that results in abnormal daily sea temperature fluctuations (Plata & Filonov, 2007), promotes non-optimal conditions that affect physiological processes with high energy demand, such as reproduction (Glynn, 2000; Glynn & D’ Croz, 1990; Santiago-Valentín et al., 2018; Spalding et al., 2001). However, gamete development has recently been documented in all reef-building scleractinian corals examined in the region (Carpizo-Ituarte et al., 2011; Chávez-Romo & Reyes-Bonilla, 2007; Glynn et al., 1991; Glynn et al., 1994; Glynn et al., 1996; Glynn et al., 2017; López-Pérez et al., 2007; Medina-Rosas et al., 2005; Rodríguez-Troncoso et al., 2011; Santiago-Valentín et al., 2015), and even the developmental stages and morphology of the larvae within adult colonies have been described (Glynn et al., 2017; Santiago-Valentín et al., 2019).
The hermatypic coral Porites panamensis Verrill, 1866 is endemic to the ETP and, as such, displays a high tolerance to a wide range of sea surface temperature and turbidity daily and seasonal fluctuations (Halfar et al., 2005; Reyes-Bonilla et al., 2007). This species is a gonochoric brooder (Carpizo-Ituarte et al., 2011; Glynn et al., 1994; Rodríguez-Troncoso et al., 2011) and is the only one for which larvae have been found in the water column in the ETP (Santiago-Valentín et al., 2019). Considering that the survival of coral reefs given anthropogenic disturbances, including the consequences of climate change, hinges on successful recruitment, and in turn, this depends on phenotypic diversity and plasticity of larvae (Roth & Deheyn, 2013). In addition, a comprehensive understanding of coral larval morphological structures suitable for settlement provides the basis for the conservation of coral populations (Aranda et al., 2011). Hence the high relevance to characterizing P. panamensis larvae through the description of histological and cytological features of the larvae during their planktonic phase. In addition, we generate data that in the future can be compared with other cnidarian larvae; this would aid in learning about specific adaptations of settlement in corals.
Materials and methods
Coral larvae were collected in Islas Marias Biosphere Reserve (21°17’24.0” N - 106°14’24.0” W). Sampling was performed in July and August 2017, recorded period of gamete maturation for P. panamensis in the region (Carpizo-Ituarte et al., 2011; Santiago-Valentín et al., 2019). The collection of larvae was performed at noon, slowly dragging a net (30 cm diameter with a mesh size of 150 µm) in the vicinity of live coral colonies for ≈20 min covering an approximate area of 250 m2. A total of six samples per month were obtained. Samples were transported to the laboratory and separated using a dissecting microscope (Carl-Zeiss®).
Morphological and molecular identification were carried over as previously described in Santiago-Valentín et al. (2019), using the molecular marker (Cytochrome oxidase subunit I gene: COXI), amplified with PCR, using the primers: LCOI490 (5´-GGGTCAACAAATCATAAAGAYATYGG -3´) and HCOI21908 (3´- TAAACTTCAGGGTGACCAAARAAYCA -5´) (Folmer et al., 1994). Forward and reverse sequences were manually edited in order to obtain a consensus sequence using Geneious® V.4.8.5 software (Biomatters, 2010). The consensus sequences were analyzed using Basic Local Alignment Search Tool (BLAST) of National Center for Biotechnology Information (NCBI). In order to determine the relationship among samples collected in other regions of Mexican Pacific and recognize the taxonomic identity of the larvae, a maximum likelihood (ML) tree with Hasegawa Kishimo Yano distances and gamma distributions was created (Kumar et al., 2016) using MEGA7®. The tree was build using, sequences from the genBank (MN005653, MN005652, MF969052, NC024182.1, KU956960.1, MN005655) and Gorgonia flabellum (GQ342418.1) was included as outgroup. The consensus sequence was deposited in the NCBI with accession number: MZ350963.
To describe the ultrastructure of the larva, each larva was individually fixed in 2.5 % glutaraldehyde in 0.2 M Millonig’s phosphate buffer (pH 7.4) and 0.14 M NaCl for 7 days, and then rinsed with phosphate buffer for 40 min; it is important to highlight that the fixation process can cause a slight shrinkage of the larvae. Post-fixation was performed in 1 % osmium tetroxide in 0.1 M sodium cacodylate buffer, with overnight washing using the same buffer. Larvae were dehydrated using an ethanol gradation series (50, 70, 80, 95, and 100 %), with infiltration in a diluted epoxy resin for 24 h (Hayat, 1986).
Thin sections (0.5 µm) were cut on an ultra-microtome (MT-x, RMC®), mounting onto glass slides, contrasted with uranyl acetate and lead citrate, and stained with toluidine blue 1 % in 0.1 M sodium borate solution at alkaline pH. Slides were first observed under Carl Zeiss AxioScope® optical microscope to assess the regionalization of structures. Electron micrographs were taken with a transmission JEOL® electron microscope (model JEM-1010) operated 60-80 kV with to resolution of 0.25 nm, and cellular structures were identified according to previous studies (Muscatine, 1974; Vandermeulen, 1974; Vandermeulen, 1975).
The total length of all planulae was determined, as well as the oral and aboral pole diameter (using photographs of larvae before the fixation process) and the length of cilia of both poles (using electron micrographs). The measures were done with the image analysis software AxioVision® (Carl Zeiss Microscopy, 2005). Data recorded are expressed as the mean ± S.D. Statistical differences in cilia length between oral and aboral poles were tested throughout t-test employing Sigma-plot V.11.
Results
Fourteen P. panamensis larvae were collected during two different sampling periods: three in July and 11 in August. The maximum likelihood tree analysis revealed that the collected larvae were clustered with P. panamensis recruits and adults of Islas Marias, Islas Marietas, and the Gulf of California (Fig. 1). The BLAST analysis showed that sequence comparisons of larvae revealed a 100 % nucleotide similarity with the species P. panamensis, which also coincides with taxonomic characteristics, concluding that the identity of the collected larvae is P. panamensis.
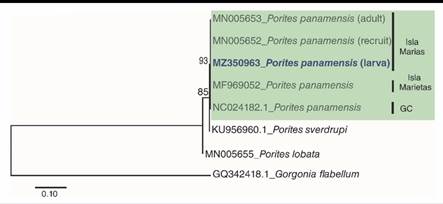
Fig. 1 Maximum Likelihood Tree of DNA secuences of partial fragment of the cytodhrome oxidase subunit I gene (COXI). Number bootstrap values 1 000 replicates. GC: Golf of California.
Larvae were fusiform in shape, and the analysis of planulae revealed typical two-layered anatomy (Fig. 2); a full ciliated translucent light brown ectoderm, and dark brown endoderm (Fig. 2A), separated by a thin acellular mesoglea (Fig. 2B). Specimens were 595.80 ± 113.57 µm in length and has an anterior pole (aboral pole; 253.84 ± 46.63 µm in diameter) that tapers to the posterior pole (oral pole; 114.97 ± 25.78 µm).
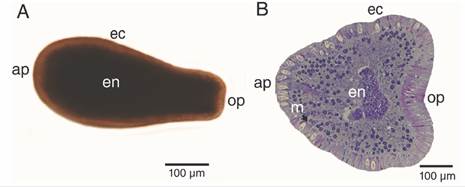
Fig. 2 Free-swimming P. panamensis larva. A. External morphology, B. longitudinal (median) section. ap: aboral pole; ec: ectoderm; en: endoderm; m: mesoglea; op: oral pole.
The ectoderm was densely covered by cilia and clusters of microvilli (Fig. 3A); the cilia showed a 9 + 2 axoneme arrangement associated to the apical-central region of the ectodermal cell and each possessed a vertically oriented rootlet (Fig. 3D). The cilia did not present differences in length (Student-t = 1.26, N = 10, P = 0.242) between oral (1.48 ± 0.11 µm) and aboral pole (1.48 ± 0.083 µm). The ectoderm was composed by heterogeneous, mono-ciliated, columnar epithelial cells. Nuclei were present as peripheral islands of heterochromatin situated at different “levels,” causing a pseudo-stratification of the epithelium (Fig. 3A, Fig. 3C). Mitochondria were observed as spherical and ovoid structures (Fig. 3C). The apical section of the ectoderm also showed semi-oval structures (Fig. 4A) with projections and other structures identified as viruses (Fig. 4B) (Vega-Thurber et al., 2017).
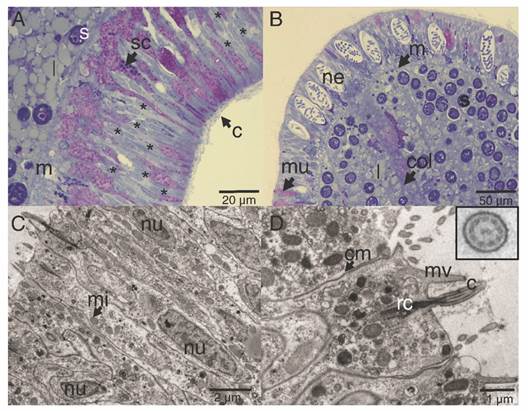
Fig. 3 Longitudinal section of the ectoderm of P. panamensis larva. A. Oral pole region stained with toluidine blue stained, B. Aboral pole region, C. Transmission electron microscopy (TEM) image of ectoderm cells of the oral pole, D. TEM image showing epithelial cells with cilia, the square is a magnification of the cross-cut of cilia. c: cilia; cm: cell membrane; col: coelenteron; l: lipids; m: mesoglea; mi: mitochondria; mu: mucus- secreting cell; mv: microvilli; ne: nematocyst; nu: nucleus; rc: root of cilia; s: symbiont dinoflagellate; sc: secretory cell.
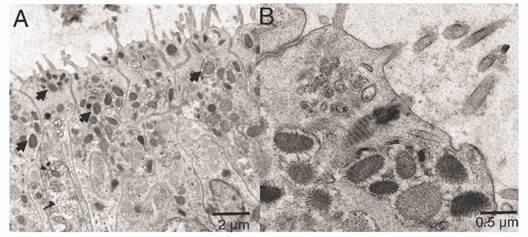
Fig. 4 Transmission electron microscopy of the ectoderm of P. panamensis larva of the semi oval structures with projections. A. Longitudinal sections of the epithelial layer with cells containing viruses. B. Close-up of some viruses (denoted by arrows).
Cnidocytes encapsulating nematocysts (Fig. 5) were most commonly associated with the ectoderm of the oral pole (Fig. 3B) and two types (shapes) of nematocysts were evidenced b-mastigophores (Fig. 5A, Fig. 5B) and isorhizas (Fig. 5C, Fig. 5D).
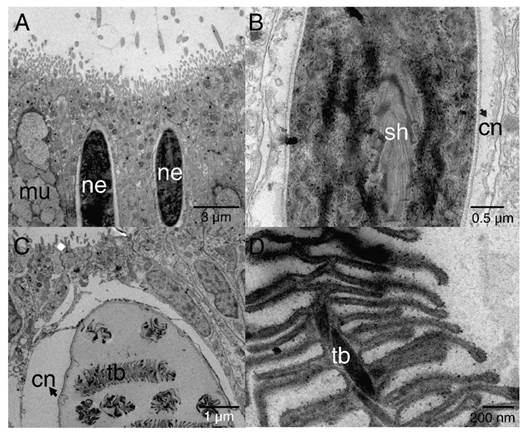
Fig. 5 TEM image showing epithelial ectoderm of the aboral pole of P. panamensis with the presence of cnidocytes. A. - B. nematocysts b-mastigophores. C. - D. nematocysts isorhizas. cn: cnida; mu: mucus- secreting cell; ne: nematocyst; sh: shaft; tb: tubule.
The highest concentrations of mucus-secreting cells (mucus polysaccharides) were observed associated with the aboral pole area (Fig. 6). The secretory cells were visualized along the ectoderm and endoderm (Fig. 6A, Fig. 6B), which differed in electron-density, shape, and size (Fig. 6D). In this study, they were classified as type I and type II, following the descriptions of Martin & Thomas (1980) and Vandermeulen (1974) (Fig. 6B). Symbionts were evident in the endoderm, and few cells in the ectoderm (Fig. 6A, Fig. 6B); micro-algae cells were surrounded by complex periplasts and lipid vacuoles. Each contained nuclei bounded by a double membrane and chromosomes attached to a prominent nucleolus. In addition, these micro-algae contained single, peripheral, multi-lobed chloroplasts, as well as pyrenoids attached to the chloroplast by one to three stalks (Fig. 6C).
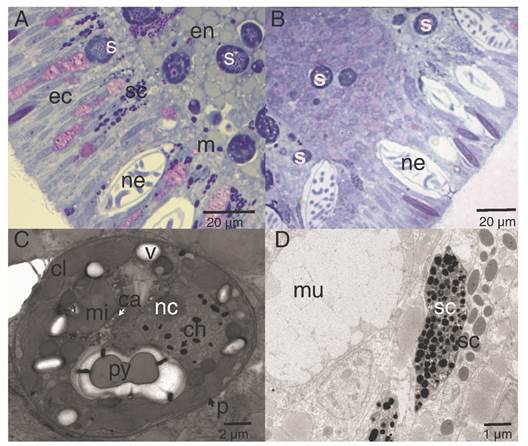
Fig. 6 Lipid and secretory structures of P. panamensis larvae. A. Median section of the endoderm and ectoderm on the lateral part of the larva. B. Aboral pole of the larva. C. TEM image of a cross-section of a symbiont dinoflagellate located in the middle region of the gastrodermis. D. TEM of a different type of secretory cell of the aboral ectoderm. ca: calcium oxalate crystal; ch: chromosome; cl: chloroplast; ec: ectoderm; en: endoderm; m: mesoglea; mi: mitochondria; mu: mucus-secreting cell; nc: nucleoli; p: cell wall; py: pyrenoid; s: symbiont dinoflagellate cell; sc: secretory cell; v: vacuole.
Discussion
The external anatomy of P. panamensis larvae share similarities with larvae of other scleractinian corals (e.g. Pocillopora damicornis in central Pacific) (Vandermeulen, 1974), Leptastrea purpurea in North Pacific (Nietzer et al., 2018), and Porites astreoides (Edmunds et al., 2001). However, differences of internal structures (e.g., the presence of symbionts, the location and development of cnidocytes, the presence of lipid vacuoles) were observed, and all are involved in their physiological performance, in response to both regional and local conditions, such as energy storage, length, and competence (pre-metamorphosis) stage. This contributes to explaining the reproductive success of P. panamensis in the region, unlike the other species present at the site (Glynn et al., 2017; López-Pérez et al., 2007; Medina-Rosas et al., 2005).
Cilia fully covered the P. panamensis larval ectoderm, with no differentiation in organization or morphology along the oral-aboral axis, the basal anatomy of the cilia is equal or similar to the majority of the cilia present in metazoan (Pitelka, 1974). The distribution of cilia in the larva allows that planulae can control over the depth, swimming actively up in a spiraling motion concerning increasing pressure even in areas with strong tidal currents (Mileikovsky, 1973; Stake & Sammarco, 2003). However, the dispersion horizontal is mostly associated with marine currents’ effect (Sammarco, 1994). Cilia provide locomotion and funciont as transductors of chemical cues (Nielsen, 1987; Nielsen 2012; Vandermeulen, 1974), influencing the dispersal and substrate selection processes, both important for larval settlement and maintenance of the population.
Two types of nematocysts were observed in the aboral pole of the planula, isorhizas, and b-mastigophores. The composition of nematocysts may vary depending on developmental stages or physiological conditions (Fautin, 1988; Fautin, 2009). The isorhizas are present in planulae but absent in adult colonies of the coral Pocillopora damicornis (Paruntu et al., 2000). Nematocysts specific (e.g isorhizas) to larvae might have functions including attachment to the substrate. When larvae detect an adequate substrate for settlement, spirocysts and isorhizas are work as a pre-attachment tool (Strömberg et al., 2019; Vandermeulen, 1974; Vandermeulen, 1975), as planulae use them as an anchoring system that latches on the substrate (Chia & Bickell, 1978). We only observed isorhizas in the collected larvae; however, the presence of spirocysts cannot be ruled out, and possibly, they develop in a more advanced stage of larva maturity or during the metamorphosis phase. The b- mastigophores has been considered as a nematocist (Mariscal, 1974), and as P. panamensis planulae is lecitotrophic and not feed during its planktonic stage, larval cnids b- mastigophores must not be used for capture prey. Thus, the b-mastigophores in planulae are expected to work as a defense system against predators, as observed in other cnidarians (Buss, 1990; Lange et al., 1992).
Also, three types of secretory cells were observed throughout the epidermis and gastrodermis. The cell mucus (mixture of polymeric glycoproteins) in the aboral epidermis they play an essential role for rapid adhesion of the coral larvae (Chia & Crawford, 1977; Vandermeulen, 1975) and, is different from the mucus in pole oral, which is characterized by its high carbohydrate content (around 80 %) (Bansil & Turner, 2006) and can also act as a stored energetic budget used during metamorphosis (Davy & Patten, 2007; Futch et al., 2010). Other secretory cells (type I, II) represent differences in the chemistry of their secretions or, are successive developmental stages of other cell types and classified as zymogen cells, which are responsible for secreting proteins (Rose & Burnett, 1968), and are located in the central region of the gastrodermis and considered as precursor cells of the specialized cells responsible for digestion in adult organisms (Haynes & Davis, 1969).
Viruses were observed in the apical zone of the epidermal cells in the larvae. Coral larvae can obtain bacteria and viruses from their progenitor, but also from the water column in early larvae and recruit stages, in fact they generally possess a far more diverse bacterial microbiomes and virus than later life stages (Van Oppen & Blackall, 2019). The viruses observed in the epidermis of the larvae apparently belong to different families including mega-virus and mini-virus (Claverie et al., 2009; Vega-Thurber et al., 2017). However, metagenomics studies are required to characterize the virus type and its possible role in early coral larvae and recruit stages.
In the gastrodermis, the presence of lipid vacuoles and symbionts was clearly evidenced. Porites panamensis larvae are non-feeding planulae lacking a mouth opening and tentacles, contrary to the planktotrophic larvae developed by spawning coral species. Contrastingly, planktonic larvae from gonochoric brooders rely on nutrients stored in their tissues, and the organic carbon traslocated through symbiosis (Muscatine & Cernichiari, 1969), and by the absorption of organics from the water column (Ben-David-Zaslow & Benayahu, 2000; Vandermeulen, 1974; Vandermeulen, 1975). The energy reserves of planulae are thus critical to their longevity and dispersal potential (Gleason & Hofmann, 2011; Harrison, 2011). Esters and triacylglycerol are the most common storage lipids in corals (Yamashiro et al., 1999); in addition, the released planulae have maternally derived endogenous lipids (up to 70 % by weight) which does not only acts as an energetic source but also contribute to the buoyancy and favors a vertical posture and displacement along the water column (Vandermeulen, 1974; Vandermeulen, 1975).
The acquisition of symbionts for P. panamensis larvae is horizontal, as they “inherit” symbiotic cells during the maturation of the oocyte (Carpizo-Ituarte et al., 2011; Rodríguez-Troncoso et al., 2011). All planktonic larvae samples evidence symbiotic cells mostly in the endoderm and, few cells in the ectoderm, later disappear from the ectoderm as the planulae matured (see Hirose et al., 2000; Hirose & Hidaka et al., 2006), as the larvae are essentially lecithotrophic upon emission and the energy translocated from the symbionts is minimal in the planulae compared with adult coral (Kopp et al., 2016).
The successful survival of larvae and the subsequent recruitment is important for the healthy maintenance of the whole community, as it contributes to population replenishment and connectivity among broadly dispersed populations. Also, the development of sensory structures is a specific key process, such as the adequate selection of substrate and its subsequent settlement, which will determine the survival of the future colony. The present study supports previous descriptions of the ultrastructure of larvae of other coral species. Structures such as secretory cells and nematocysts are abundant in the aboral pole, which suggests their importance in the substrate exploration larval settlement.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


