Introduction
The natural ecosystems of the northern region of Mato Grosso (Brazil) are in process of fragmentation, mainly due to population growth and the expansion of agricultural frontier. As a consequence, the formation of mosaics of remaining vegetation is increasing, reducing the size of the populations and causing changes in the ecological and genetic processes of the natural species that occur there (Dardengo et al., 2021).
This exploitation contributes to the degradation of the environment and has become a worrying factor for the preservation of the species Euterpe precatoria Mart. (açaí). This species occurs in an Amazonian environment and has high commercial interest, being used by Amazonian people for civil construction and for food, both the fruit for juices and ice cream, and the heart of palm (Arruda et al., 2014).
The illegal logging of adult individuals of E. precatoria to remove the heart of palm and wood, causes a reduction in the number of individuals, thus, alleles of interest are lost and not transmitted to the next generations (Azêvedo et al., 2017). Knowing the distribution of genetic diversity among and within natural populations is very important for the development of conservation strategies. In addition, it allows a better understanding of how the selection is working, therefore, a widely used way to detect this variability is through studies of the genetic structure of natural populations (Estopa et al., 2006). Genetic studies with native plant species must be carried out to gather information that contributes to in situ conservation, sustainable management and the formation of seed collection areas, in order to recover degraded areas (Pinto et al., 2009). Molecular markers have been used frequently in studies like these (Ramos et al., 2021).
Among the molecular markers used in studies about forest fragmentation, microsatellites or Simple Sequence Repeat-SSR stand out, which are codominant and specific to each species, with possible transferability to other species of the same genus (Faleiro, 2007). SSRs are markers widely used to estimate genetic parameters of populations, gene flow patterns and kinship, being abundant and well distributed across the plant genome. According to Manel et al. (2003), the use of molecular data in studies of population and landscape genetics, contributes to the analysis of the gene flow rate, genotype distribution, genetic adaptation, adaptive differentiation and speciation. Thus, we aim to assess the diversity and genetic structure of native populations of E. precatoria, located in micro-region II-North, State of Mato Grosso, Brazil, to support strategies for managing the genetic resources of the species for its conservation or domestication.
Materials and methods
Study area and Sampling: The study was carried out in five municipalities (Alta Floresta, Carlinda, Nova Bandeirantes, Nova Monte Verde and Paranaíta) that compose the planning micro-region II-North of the state of Mato Grosso, Brazil. This area is a natural landscape that once was a unique forest, and it was selected for the study because there is a lot of deforestation pressure.
For the analysis of genetic diversity and structure, leaf material was collected from 106 adult individuals (individuals in phase of fructification) of E. precatoria distributed in five populations located in the municipalities. Sampling was carried out randomly in the forest fragment, each individual sampled was an average of fifty meters apart.
DNA extraction and Polymerase chain reaction (PCR) amplification: The total genomic DNA was extracted from approximately 100 mg of leaves, using the CTAB method described by Doyle and Doyle (1990), with modifications suggested by Soares et al. (2016) and Zortéa et al. (2016). The DNA concentration were estimated by comparison with the lambda DNA and the samples were diluted to a concentration of 15 ng.μL-1 to perform the amplifications.
Seven isolated microsatellite primers (SSR) that were characterized by Gaiotto et al. (2001) were tested in an initial PCR amplification using three E. precatoria individuals. Of the seven SSR primers tested, five amplified the E. precatoria genome and were selected for genetic diversity analysis.
The amplification reactions via polymerase chain reaction (PCR) were performed with a final volume of 13 μL: 0.12 μL of Taq DNA polymerase (5 U / μL); 1.5 μL 10x Buffer [10 mM Tris-HCl (pH 8.3); 50 mM KCl; 0.1 % tween; 10 mM MgCl2]; 1.04 μL MgCl2 (25 mM); 2 μL of primer R and F (2 μM); 1.5 μL of dNTPs (1 mM); 2 μL of DNA (30 ng); completing the volume with ultrapure Milli-Q water autoclaved.
The amplifications were carried out in a Biocycler thermocycler under the following conditions: an initial denaturation cycle at 94 ºC for 15 minutes, followed by 35 cycles: a) denaturation at 94 ºC for thirty seconds; b) annealing at 58-63 °C (depending on the primer used) for 1 minute and 30 seconds; c) extension of 72 ºC for 1 minute and a final extension cycle of 72 ºC for 7 minutes (Aguilar-Barajas et al., 2014).
The amplification products were separated by electrophoresis on 3 % agarose gel (mv-1) in running buffer TBE 1X (EDTA 0.02 M; boric acid 0.89 M; Tris-base 0.89 M), in constant voltage of 70 V for approximately four hours, to avoid overlap of the amplification products. The fragments were scored using GelQuantPRo®.
Data analysis: We used the Power Marker program (Liu & Muse, 2005) to assess allelic frequency, genetic diversity, the observed and expected heterozygosity, fixation index and the polymorphism information content (PIC). Nei (1983) matrix of genetic distance between E. pracatoria trees was estimated using the same program. This matrix was imported by MEGA 3.1 to construct a dendrogram of mean distance using the unweighted pair group method with arithmetic mean (UPGMA). The presence of null alleles was check by the use of Micro-Cheker program (Van Oosterhout et al., 2004).
The Structure program (Pritchard et al., 2000), which is based on Bayesian statistics, was used to indicate the number of genetic groups (K). We conducted 20 runs for each K value, with 250 000 burn-ins and 500 000 Markov chain Monte Carlo simulations. To determine the most probable value of K, we used the criteria proposed by Evanno et al. (2005).
The most probable number of different populations that contributed to the genetic composition of the E. precatoria populations under study was verified by the ΔK estimate described by Evanno et al. (2005). The ΔK was estimated for K ranging from one to eight, with the highest value occurring for the number of genetically distinct populations that best explains the data set.
Principal coordinate analysis (PCoA), deviations of the Hardy-Weinberg equilibrium, analysis of molecular variance (AMOVA) and the analysis of rare (AR) and exclusive alleles (EA) were performed using the GenAlEx 6.5 program (Peakall & Smouse, 2006).
Results
Populational Genetic Diversity of Euterpe precatoria: The microsatellite loci showed a total of 30 alleles in E. precatoria, ranging from 5 to 7 alleles, with an average of 6 alleles per locus. The content of polymorphic information (PIC) was above 0.60 for all loci (Table 1). The average expected heterozygosity (He) was higher than the expected heterozygosity (Ho), with a higher frequency of homozygotes in the sampled E. precatoria populations.
Table 1 Genetic parameters for five microsatellite loci, based on the amplification of the DNA of 106 Euterpe precatoria individuals from five populations sampled in the micro-region II-North in the State of Mato Grosso, Brazil.
| Loci | Na | He | Ho | f | PIC |
| EE2 | 07 | 0.73 | 0.06 | 0.91* | 0.70 |
| EE15 | 07 | 0.83 | 0.05 | 0.94* | 0.80 |
| EE23 | 05 | 0.76 | 0.11 | 0.85* | 0.72 |
| EE43 | 05 | 0.70 | 0.00 | 1.00* | 0.65 |
| EE54 | 06 | 0.82 | 0.64 | 0.22* | 0.79 |
| Total | 30 | - | - | - | - |
| Average | 06 | 0.76 | 0.18 | 0.77* | 0.74 |
Number of alleles (Na), Expected heterozygosity (He), Observed heterozygosity (Ho), Fixation index (f) and Polymorphic Information Content (PIC). *P < 0.05.
The fixation index had an average of 0.77 and all loci showed positive fixation index, with locus EE43 presenting f = 1, in other words, this locus did not reveal any heterozygote in the populations (Table 1), confirming the low heterozygosity and the high inbreeding in the studied E. precatoria populations.
Nei’s genetic distance (Nei, 1972) revealed that the NMV and AF populations are the most dissimilar, that is, the most genetically different, being geographically distant at approximately 105 km. The PR and NMV populations showed the greatest genetic similarity and were approximately 87 km apart, indicating a greater possibility of flow between the sampled populations (Table 2).
Table 2 Geographical distance (above the diagonal) in kilometers (km) and Nei’s Genetic Distance (1983) (below the diagonal) among the populations of Euterpe precatoria sampled in the microregion II-Norte, in the State of Mato Grosso, Brazil.
| Populations | AF | CR | NB | NMV | PR |
| AF | - | 73.044 | 150.557 | 104.999 | 86.811 |
| CR | 0.584 | - | 213.678 | 166.383 | 110.077 |
| NB | 0.2778 | 0.246 | - | 48.6 | 127.97 |
| NMV | 0.060 | 0.440 | 0.445 | - | 86.811 |
| PR | 0.243 | 0.381 | 0.605 | 0.706 | - |
AF: Alta Floresta; CR: Carlinda; NB: Nova Bandeirantes; NMV: Nova Monte Verde; PR: Paranaíta.
Table 3 shows the results of genetic diversity by population. The PR population was the one with the highest number of alleles, followed by the populations of NMV and NB. The analysis by loci and the analysis by populations showed that the number of homozygotes was greater than that of heterozygotes. The fixation index was positive for all populations, which confirms the greater number of homozygotes and the low frequency of heterozygotes in the sampled populations. The AF population showed the lowest He (0.441) and f (0.537) values and the highest heterozygosity observed. It was not observed any null allele in the analysis.
Table 3 Estimation of the genetic diversity parameters of five populations of Euterpe precatoria naturally occurring in the micro-region II-North, State of Mato Grosso, Brazil.
| Population | N | A | H e | H o | f |
| AF | 21 | 15 | 0.441 | 0.207 | 0.537 |
| CR | 19 | 15 | 0.564 | 0.200 | 0.652 |
| NB | 23 | 17 | 0.626 | 0.156 | 0.754 |
| NMV | 20 | 19 | 0.563 | 0.180 | 0.686 |
| PR | 23 | 21 | 0.598 | 0.130 | 0.785 |
| Average | 21.2 | 17.4 | 0.559 | 0.175 | 0.692 |
AF: Alta Floresta; CR: Carlinda; NB: Nova Bandeirantes; NMV: Nova Monte Verde; PR: Paranaita N = Number of individuals; A = Allelic richness; He = expected heterozygosity; Ho = observed heterozygosity; f = Intrapopulational fixation index.
Genetic structure and populational differentiation of E. precatoria: The dendrogram generated from the genetic distance of Nei (1983) by the UPGMA method formed two groups. Group 1 (G1) was formed by the populations of Alta Floresta (AF) and Carlinda (CR), while in group 2 (G2) the populations of Nova Bandeirantes (NB), Nova Monte Verde (NMV) and Paranaíta (PR) were allocated. In G2, two subgroups are formed, the first subgroup formed by the population of NB and a second subgroup formed by the populations of NVM and PR, the geographic distance may have been the factor that contributed to the similarity between these populations, since they are neighboring municipalities (Fig. 1A, Fig. 1B).
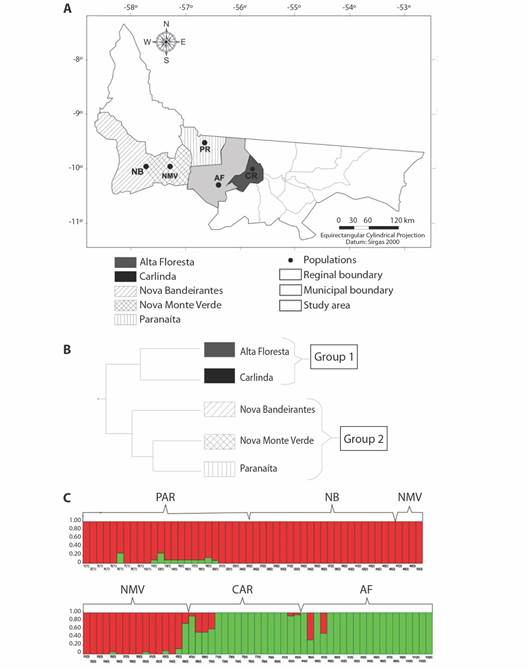
Fig. 1 A. Geographical location of the five populations of Euterpe precatoria under study in micro-region II-North in the State of Mato Grosso, Brazil. B. Dendrogram obtained by the UPGMA method, based on the genetic distances of Nei (1983). Branch reliability was tested by bootstrap analysis using 1 000 replications. C. Grouping of 106 individuals from five populations of Euterpe precatoria based on five SSR loci using the “Structure” Program. Individuals are represented by vertical columns and are shaded according to their group (two genetic groups, K = 2). PAR: Paranaíta; NB: Nova Bandeirantes; NMV: Nova Monte Verde; CAR: Carlinda and AF: Alta Floresta.
The Bayesian analysis performed by the “Structure” program corroborates the result obtained by the UPGMA method, with the formation of two distinct groups (k = 2). The two genetic groups found in the evaluated sample are represented in black (group 1) and gray (group 2), indicating that the populations of PR, NB and NMV are constituted mainly by the genetic group 1, while the populations of AF and CR by group 2 (Fig. 1C).
The PCoA explained 29.79 % of the total variation, with 18.06 % for the first component, 11.73 % for the second (Fig. 2).
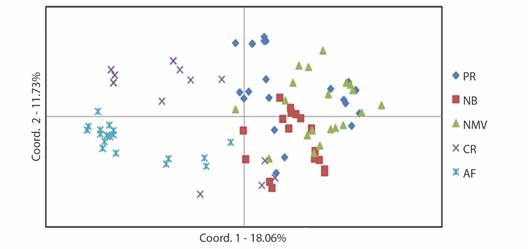
Fig. 2 Graphic dispersion from the analysis of the main coordinates of 106 individuals of Euterpe precatoria from sampled populations in microregion II-Norte, State of Mato Grosso, Brazil. AF: Alta Floresta; CR: Carlinda; NB: Nova Bandeirantes; NMV: Nova Monte Verde; PR: Paranaita. Coord.: Coordinate.
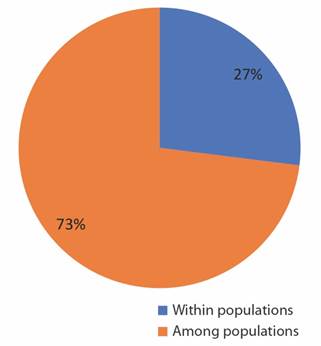
As in the other clusters, we observe the formation of two groups and the individuals were allocated according to the previous groupings generated by UPGMA (Fig. 1B) and by Structure (Fig. 1C). AMOVA revealed that 73 % of the total variance occurred within populations and 27 % among populations (Fig. 3).
Discussion
In the work of Oliveira et al. (2010), with E. oleracea, 42 alleles were found for the set of five primers used, ranging from 10 alleles (EE23) to 04 alleles (EE2), values higher than those found in this study, while Azêvedo et al. (2017) found 08 alleles, with an average of 1.6 alleles per locus, for E. precatoria, a number lower than that found in this study, that is, the data found in this study are within the expected for the species.
All loci analyzed in this study were highly informative, confirming the transferability of the microsatellites from E. edullis to E. precatoria. According to Botstein et al. (1980) Polymorphism Information Content (PIC) values below 0.25 are considered uninformative, those that reveal values between 0.25 and 0.50 are classified as moderately informative and above 0.50 highly informative. Oliveira et al. (2010) when transferring the same markers from E. edullis to E. oleracea found the PIC value of 0.86 for primers EE23 and EE54, highly informative values.
In this study, all loci presented f values above 0.22, indicating excess of homozygotes in the populations, according to Azevedo et al. (2007), the fixation index value varies between -1 and +1, positive values indicate excess homozygote, negative values excess of heterozygotes and zero is the value found for a population in Hardy-Weinberg equilibrium.
Among the results found, this study highlights the low heterozygosity and the high rate of inbreeding in all loci, which can be influenced by the low flow of dispersers and also by the reducing number of E. precatoria individuals in the fragments. Another explanation for the high inbreeding values lies on the presence of null alleles, because it increases the number of homozygous individuals, since just one of the alleles amplifies itself in cases of null allele in heterozygous plant (Nybom, 2004), however, it was not found any null allele in the analysis.
The forest fragmentation found in the field can be one of the main factors for the high rate of inbreeding, since fragmentation becomes an “obstacle” for the migratory flow of animal species that provide systemic services to the plant, such as dispersers. The E. precatoria fruits are part of the diet of several animals that are seed dispersers in the forest, the main wild animals that help in the dispersion of the species are birds of the Psittacideae, Ramphastidae and Cracidae families (Rocha & Viana, 2004). Another factor that may also have contributed to inbreeding is the species life strategy, which forms a seedling bank close to the mother plant (Oliveira et al., 2010; Ramos et al., 2021), in the other hand, studying the same species, have found a high expected heterozygosity, which according to the authors, is explained by the allogamy. So once again, we can say that the fragmentation may be the cause for this increase of homozygous levels.
Inspite, of the low number of sampled individuals, the presence of rare (PR, NMV and CR population) and exclusive alleles (NMV population), indicated the importance of these populations, thus, they need preservation strategies for specimen conservation. Dias Filho (2006) in a study with the species Eutepe edullis identified seven exclusive alleles in a natural population in the Municipal Reserve of Santa Genebra, São Paulo State, using the same set of microsatellites tested in this work and used this information to justify the conservation of the species in the area.
For this study, the K value indicated the presence of two distinct genetic groups, or populations, among the individuals studied, showing that even with the geographical distance and fragmentation in the landscape, there is still a connection among the populations, because there are some corridors of vegetation among them.
The values presented for E. precatoria in this study, indicated the existence of greater variability within populations, a common fact for allogamous species. Oliveira et al. (2010) also identified high genetic differentiation (69.88 %) within natural populations of E. oleracea, corroborating the values obtained for natural populations studied in this work.
The populations studied present levels of gene diversity, high average number of alleles per locus and presence of rare and exclusive alleles. It is also important to infer that the development of agricultural activities that has been occurring in an accelerated manner around the forest fragments will provide an increase in the isolation of these populations and loss of these alleles, so the establishment of permanent conservation units with corridors among them, could be a valuable tool to preserve genetic diversity among the individuals of these natural populations. We strongly suggest more studies with the species, with more loci and individuals sampled on it.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio