Introduction
The order Cassiduloida (sensuSouto, Mooi, Martins, Menegola, & Marshall, 2019) includes the families Cassidulidae, Eurhodiidae, Faujasiidae, Neolampadidae, and Pliolampadidae (Souto et al., 2019), is represented by 800 species, most of them fossils and a few living ones (Kier, 1962). One of the most characteristic aspects of these families is that their fossil record, and not the living representatives, contains most of their morphological diversity. Their variable morphology has made their taxonomic study complicated and possibly denotes that the group is in the process of extinction (Suter, 1988).
The cassiduloids first appeared and changed from infaunal to epifaunal habits during the Lower Jurassic (Boivin, Saucède, Laffont, Steimetz, & Neige, 2018; Souto et al., 2019). They diversified in the Early Cretaceous and survived the K-Pg mass extinction, were common in the Late Cretaceous and Early Cenozoic, being more successful in the Eocene (> 40 % of the echinoid diversity), and finally have dramatically declined in number since then (Kier, 1962; Kier, 1974; Suter, 1988; McNamara, Pawson, Miskelly, & Byrne, 2017). Kier (1962) noticed several morphological changes within the cassiduloids: abrupt reduction from two pores to one pore in each ambulacral plate beyond the petal and the introduction of buccal pores (Cenomanian), due to a radical change in the living habits (began to borrow shallowly into the substratum); and a change in the structure of the apical system from tetrabasal to monobasal (Maastrichtian), probably produced by parallel mutations and parallel selections. Recent studies have shown that their evolutionary history has been dominated by high levels of homoplasy and a dearth of unique, novel traits (Souto et al., 2019).
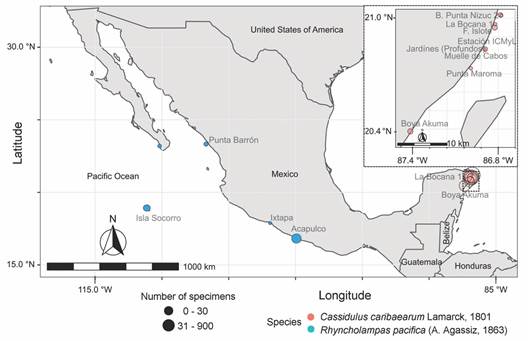
In Mexico, there exist two living species of cassiduloids: Cassidulus caribaearum and Rhyncholampas pacifica (Buitrón-Sánchez, Solís-Marín, Conejeros-Vargas, & Caballero-Ochoa, 2019). Cassidulus caribaearum inhabits warm, shallow waters (26-28 °C), from less than 1 to 18 m in depth, buried up to 20 cm in calcareous sand (± 2 000-44 μm) or carbonate substratum of the tropical Atlantic West coast, and it is probably endemic to the Caribbean Sea (Fig. 1) (Kier, 1975; Gladfelter, 1978; Borrero-Pérez, Benavides-Serrato, & Diaz-Sanchez, 2012; Solís-Marín et al., 2013; Solís-Marín, Caballero-Ochoa, Laguarda-Figueras, & Durán-González, 2017; Souto & Martins, 2018). Rhyncholampas pacifica lives in warm, shallow waters (26-30 °C) of the Tropical Eastern Pacific Ocean (Fig. 1). This species lives gregariously and partially buried to the level of the petaloids on sandy beaches at a depth of 2 to 130 m (Agassiz, 1872; Clark, 1925; Mooi, 1990; Solís-Marín et al., 2013; Caballero-Ochoa, Martínez-Melo, Conejeros-Vargas, Solís-Marín, & Laguarda-Figueras, 2017; Schultz, 2017).
Morphometrics evaluates the size and shape variation of biological forms through statistical analysis (Ocakoglu & Ercan, 2013). The traditional approach involves two-dimensional linear measurements such as lengths, widths and distances, and angles or ratios; it has been used in taxonomy since it is useful for making morphological comparisons and establishing specific boundaries, as well as assessing growth changes (Ocakoglu & Ercan, 2013; Remagnino, Mayo, Wilkin, Cope, & Kirkup, 2016; MacLeod, 2017). The application of morphometrics on cassiduloid taxonomy dates back to McKinney (1986), who discussed the heterochronic-ecological relationships between fossil irregular echinoids, including Rhyncholampas species. Although Carter and Beisel (1987) did not perform any statistical analysis, they also considered width/length ratios of the test for separating Eurhodia, Rhyncholampas and Cassidulus species. In addition, Ciampaglio and D’Orazio (2007) and Martínez-Melo (2008) provided insights into the growth trajectories and heterochronic processes between Eurhodia appendiculata, Rhyncholampas carolinensis and Eurhodia rugosa, and between C. caribaearum and R. pacifica, respectively. Recently, Martínez-Melo, De Luna and Buitrón-Sánchez (2017) evaluated the contours of the tests (lateral, aboral and posterior) of Cassidulidae species through geometric analyses, being the first study focused on this computational approach for cassiduloids.
The test shape is the most important characteristic to distinguish between species of cassiduloids (Souto et al., 2019). In most of the studies, the morphological diversity of the order Cassiduloida has been described (Souto et al., 2019); however, the intraspecific variation of the morphological characters of the recent cassiduloids in Mexico has not been evaluated. The objective of this work is to compare the basic morphological and morphometric aspects, as well as to evaluate the intraspecific variation of the morphometric characters of R. pacifica and C. caribaearum.
Materials and methods
Data collection: A total of 2 158 specimens of recent cassiduloids were examined: C. caribaearum and R. pacifica; these are housed at Colección Nacional de Equinodermos “Dra. Ma. Elena Caso Muñoz” (ICML-UNAM) in Mexico (Appendix 1).
Morphometric analyses: We selected 50 specimens of different sizes of C. caribaearum and R. pacifica, from 3.3 to 51 mm in length; these were randomly selected and were photographed from the aboral, oral and lateral views. ImageJ software was used to obtain nine measurements for each specimen: TLa, TW, Da-ppa, TLo, Da-pta, THl, TWl, PpL, and PpW (Fig. 2, Table 1). A linear regression analysis was performed to test the relationship between the length and width of the test in GraphPad Prism. A Principal Component analysis using Primer-6 software was performed; five ratios were considered: 1) test length (aboral view)/test width (at the level of the apical system), 2) test length (aboral view)/distance from the ambitus to the periproct apex, 3) test length (oral view)/distance from the ambitus to the peristome apex, 4) test height (lateral view)/test length (lateral view), and 5) periproct length/periproct width. The data were transformed through square roots (Lawrence & Cobb, 2017) (Table 1).
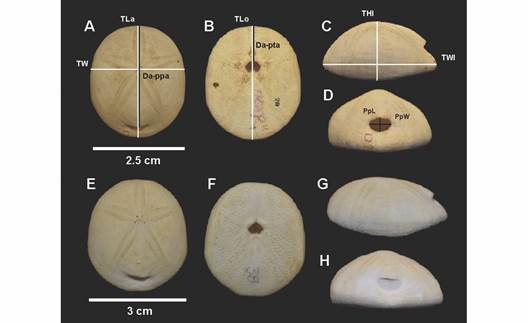
Fig. 2 Rhyncholampas pacifica, ICML-UNAM 4.48.3. A. Aboral view. B. Oral view. C. Lateral view. D. Posterior view. Cassidulus caribaearum, ICML-UNAM 4.96.6. E. Aboral view. F. Oral view. G. Lateral view. H. Posterior view. Abbreviations refer to measurements defined in Table 1.
Table 1 Abbreviations and definitions of measurements and ratios used in this study
| Abbreviation | Definition |
| TLa | Test length (aboral view) |
| TW | Test width (at the level of the apical system) |
| Da-ppa | Distance from the ambitus to the periproct apex |
| TLo | Test length (oral view) |
| Da-pta | Distance from the ambitus to the peristome apex |
| THl | Test height (lateral view) |
| TWl | Test width (lateral view) |
| PpL | Periproct length |
| PpW | Periproct width |
| A | Ambitus |
| PW | Peristome width |
| PL | Peristome length |
| AIIIL | Third ambulacrum length |
| AIL | First ambulacrum length |
| MAW | Maximum width ambulacra |
| PIW | Maximum width of the outer poriferous zone of petal I |
| PB | Petaloid beginning (%) |
| AFFP AFTP | Angle between the first and fifth petaloids (°) Angle between the first and third petaloids (°) |
| TLa / TW | Test length (aboral view) / Test width (at the level of the apical system) |
| TLa / Da-ppa | Test length (aboral view) / Distance from the ambitus to the periproct apex |
| TLo / Da-pta | Test length (oral view) / Distance from the ambitus to the peristome apex |
| THl / TWl | Test height (lateral view) / Test width (lateral view) |
| PpL / PpW | Periproct length / Periproct width |
| PBS | Peristome base shape (rect, rounded, trapezoidal and triangular) |
| ASP | Apical system position (subcentral, lateral) |
| PL / PW | Peristome length / Length of posterior side of peristome |
| AIL / AIIIL | Ambulacrum I length / Ambulacrum III length |
| MAW / PIW | Maximum ambulacral I width / Maximum width of the outer poriferous zone of petal I |
Additionally, we randomly selected 62 other specimens of C. caribaearum and R. pacifica in order to have representatives from other localities and to consider all possible sizes; and to observe which characters yield significant information for species identification. Eleven traits (TWl, THl, PW, PL, AIL, AIIIL, MAW, PIW, PB, AFFP, AFTP) were measured three times each, using an electronic Vernier caliper. We also considered two qualitative data: the peristome base shape and apical system position. All measurements, qualitative data, and ratio abbreviations are detailed in Table 1 (Fig. 3).
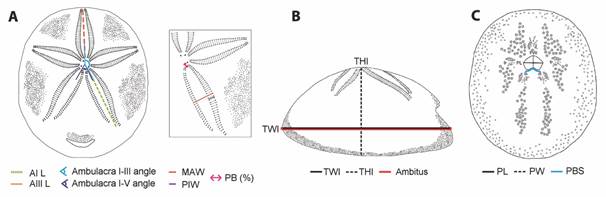
Fig. 3 Measurements used in the analysis. A. Aboral view and ambulacra I and II. B. Lateral view. C. Oral view. Abbreviations refer to measurements defined in Table 1.
The Pearson’s Correlation Coefficient was used to identify the greatest number of significant correlations between the characters of the species R. pacifica and C. caribaearum. A distribution analysis was performed to analyze the shape of the peristome using CRAN R’s factoextra and FactoMineR packages (Lê, Josse, & Husson, 2008; R Core Team, 2019). To compare the average values between different measurements, a F-test was run to check that there were similar variances between the species; the results of these F-test were then used in t-tests to analyze whether there are specific differences. Normality of measurements was verified by a Shapiro-Wilk test. We considered a test with a P-value < 0.05 to be statistically significant.
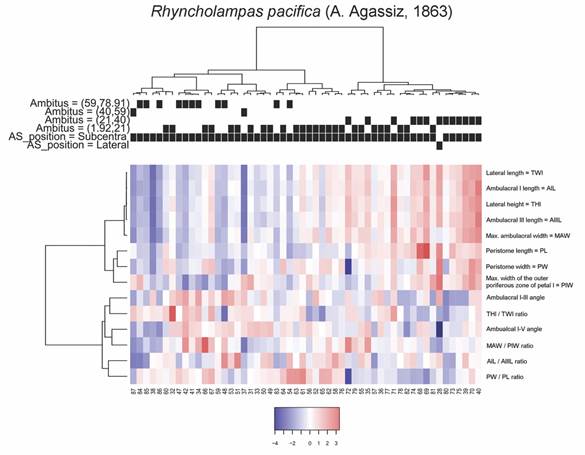
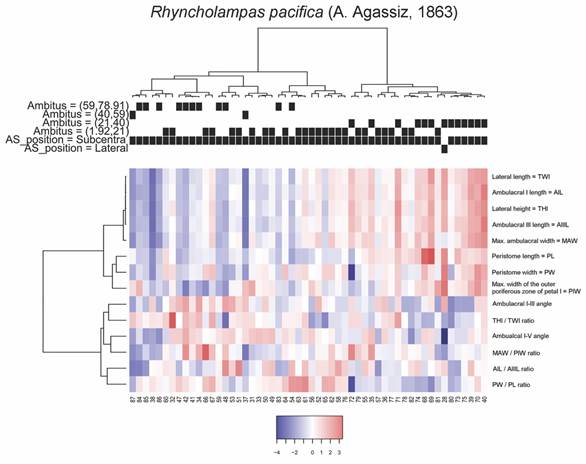
To determine whether morphological groups exist within the analyzed species, a cluster analysis was carried out using Ward’s minimum variance method. For this analysis, all the data from the specimens were used, and it was found that in both species the topology of the variables presents a similar ordering. To define the number of groups, the “Average Silhouette” method of the fviz_nbclust function, included as part of CRAN R’s factoextra and FactoMineR packages (Lê et al., 2008; R Core Team, 2019) was used. This also allowed us to report the average values of the non-standardized numerical variables by group, and to decide what separation distance to accept between different clusters.
Linked to the “Average Silhouette”, k-mean analysis was performed to check if there were differences between the parameters in assigned groups. These analyses use the algorithm of Hartigan and Wong (1979), assigning the variables to the fixed number of clusters.
Results
Evaluation of test length and width: The linear regression (Fig. 4) showed a positive correlation between the variables Test length (aboral view) and Test width (at the level of the apical system). In this regard, R. pacifica reaches test length values of 51.5 mm and test width values of 42.6 mm, whereas C. caribaearum does not exceed sizes of 22.9 mm and 19.4 mm.
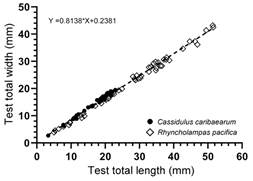
Fig 4 Linear regression of Test width (at the level of the apical system) vs Test length (aboral view) between Rhyncholampas pacifica and Cassidulus caribaearum.
The eigenvalues for PC1 and PC2 were 1.21×10-2 and 4.41×10-3, whereas the percentages of variation were 59.3 % and 21.6%, respectively (Table 2); therefore, both PC1 and PC2 explain 80.9 % of the cumulative variation of the data. The greatest eigenvalues for the first component occurred in THl/TWl and PpL/PpW, whereas in the second component, TLo/Da-pta and THl/TWl had the greatest eigenvalues. Considering both components, the THl/TWl (0.917) and TLo/Da-pta (0.925) were the two ratios which most contribute to the variation of the data, and separation of R. pacifica from C. caribaearum (Fig. 5).
Table 2 Eigenvalues (Principal Components Analysis) of Rhyncholampas pacifica and Cassidulus caribaearum
| Eigenvalues | Principal Component | Eigen-values | Explained variation (%) | Cumulative variation (%) | ||
| PC1 | 1.21×10-2 | 59.3 | 59.3 | |||
| PC2 | 4.41×10-3 | 21.6 | 80.9 | |||
| PC3 | 2.3×10-3 | 11.3 | 92.2 | |||
| PC4 | 1.21×10-3 | 5.9 | 98.1 | |||
| PC5 | 3.89×10-4 | 1.9 | 100 | |||
| Eigenvectors | Variable | PC1 | PC2 | PC3 | PC4 | PC5 |
| TLa / TW | -0.138 | -0.011 | 0.212 | -0.224 | 0.941 | |
| TLa / Da-ppa | 0.020 | -0.085 | 0.370 | -0.878 | -0.290 | |
| TLo / Da-pta | -0.366 | 0.925 | 0.001 | -0.077 | -0.061 | |
| THl / TWl | 0.197 | 0.364 | -0.053 | -0.081 | 0.132 | |
| PpL / PpW | 0.079 | 0.058 | 0.903 | 0.408 | -0.094 | |
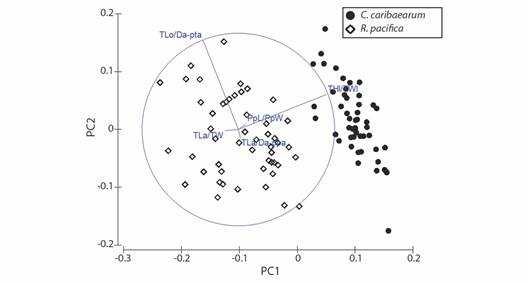
Fig. 5 Ordination (Principal Components Analysis) of Rhyncholampas pacifica and Cassidulus caribaearum. See Table 1 for acronyms.
Morphometric evaluation of other characters of taxonomic importance: In R. pacifica the highest Pearson´s Correlations Coefficients were found between PW and PL, the lengths of ambulacra I and III; and MAW and PIW. Pearson’s Correlation Coefficient values for this species ranged from 0.71 (test width (lateral view) vs. length of ambulacrum III; peristome length vs. peristome width and 0.99 (test width (lateral view) vs. lengths of ambulacra I/III and test height (lateral view)). In the case of C. caribaearum, the strongest correlations had values of 0.60 (length of ambulacrum I vs. length of peristome) and 0.94 (test width (lateral view) vs. test height (lateral view)).
When analyzing the peristome base shape along with PW and PL, it was found that, in R. pacifica, it was wider and higher and the shape was triangular, while in C. caribaearum, the most predominant shape was round (Fig. 6).
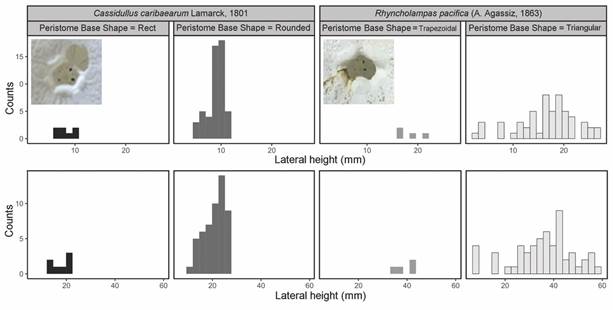
Fig. 6 Peristome base shape distribution analysis of Cassidulus caribaearum and Rhyncholampas pacifica.
In the specimens of R. pacifica, the largest correlation values ranged between 0.45 and 0.50, while in C. caribaearum the correlation values were between 0.40 and 0.45, which indicates a weak correlation (Fig. 7). When comparing the average values of the relationships between the lateral height and length for both species, a significant difference was found between the analyzed species (F test for variances: F = 0.5072, g.l.numerator = 59, g.l.denominator = 58, P = 0.0104; t-test: t = 6.2751, d.f. = 104.69, P = 8.022×10-9).
When linear regression models were used to compare the relationships of TWl and PW with respect to other variables as possible intraspecific differentiators, no significant covariations were found. Covariates that were analyzed and showed values of lower significance were: 1) PBS (estimated parameter = 1.7148, S.E. = 1.2320, t = 1.392, P = 0.17), 2) THl/TWl ratio, and 3) PBS (estimated parameter = -4.3054, S.E. = 2.7814, t = 1548, P = 0.128).
Relationships between THl vs. TWl of R. pacifica tend to be slightly lower in the specimens with an ambitus between 21 and 40 mm; this was also verified with a regression model (estimated parameter Ambitus21-40 = -0.2008, S.E. = 0.8861, t = -2.266, P = 0.0276). This observation should be tested further with a larger sample to determine if it occurs in a general way in the species. Cassidulus caribaearum specimens with larger ambitus perimeter sizes (> 40 mm) tended to present a relationship between TWl and PW independent of the relationship between THl vs. TWl, while specimens of smaller size (up to 40 mm perimeter) tended to have a PL/PW ratio that correlates positively with the relationship between THl vs. TWl (Fig. 7); however the increased ratios were not significant (P = 0.170). In R. pacifica, some of the forms that had higher PL/PW ratios also had elevated and triangular peristome base shapes. In addition, the specimens that had perimeters of the ambitus between 40 and 59 mm generally tended to present a lower PL/PW ratio as the first of these relationships decreases (Table 3); there was also no significant correlation (P = 0.0991).
Table 3 K-means (Silhoutte) cluster analysis in Cassidulus caribaearum (Dissimilar averages are marked in bold. Minimum and maximum averages are marked in black)
| Variable | SW test P-value | Centers (Average) | ANOVA P-value | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |||
| Lateral length = TWl | 0.0579 | 16.155 | 24.484 | 22.567 | 24.532 | 0.162 |
| Lateral height = THl | 0.0439 | 7.1400 | 10.315 | 9.6115 | 10.810 | 0.324 |
| THl/TWl ratio | 0.0591 | 0.4424 | 0.4212 | 0.4280 | 0.4423 | 0.222 |
| Peristome length = PL | 0.4174 | 1.6975 | 2.2000 | 1.8321 | 2.1550 | 0.625 |
| Peristome width = PW | 0.0539 | 1.5550 | 2.0600 | 1.9029 | 1.9675 | 0.256 |
| PW/PL ratio | 0.0316 | 0.9349 | 0.9519 | 1.0691 | 0.9592 | 0.117 |
| Ambulacral I length = AIL | 0.3911 | 5.6587 | 9.1062 | 8.2250 | 9.5600 | 0.368 |
| Ambulacral III length = AIIIL | 0.8939 | 7.4375 | 11.629 | 10.205 | 12.212 | 0.696 |
| AIL/AIIIL ratio | 0.1204 | 0.7714 | 0.8131 | 0.8173 | 0.7845 | 0.429 |
| Ambulacral I-III angle | 0.1449 | 66.250 | 63.750 | 70.429 | 73.750 | 0.926 |
| Ambulacral I-V angle | 0.0297 | 64.750 | 60.000 | 67.714 | 77.250 | 0.069 |
| Max. ambulacral width = MAW | 0.8554 | 1.8562 | 3.6925 | 2.5100 | 2.8100 | 0.345 |
| Maximum width of the outer poriferous zone of petal I = PIW | 0.6090 | 0.4500 | 0.4837 | 0.4014 | 0.5750 | 0.015 |
| MAW/PIW ratio | 0.0010 | 4.2321 | 5.9318 | 6.8442 | 4.9714 | 0.008 |
| Size of group | 8 | 8 | 14 | 4 | ||
| Within Sum of Squares | 176.9432 | 246.7969 | 387.0671 | 66.1314 | ||
| Between_SS / Total_SS | 63.50 % | |||||
To describe other morphometric features, we analyzed the distributions of the angle between the ambulacra I and V vs. the THl/TWl ratio. In this case, we recognized two patterns: 1) The larger specimens of C. caribaearum (ambitus 59-78.1 mm) had larger angles and larger distances between ambulacra, from 59-79 mm long and more than 70° (acute angle), with straight and rounded peristome base shape, and were independent from the test ratio compared with the rest of the sizes; and 2) In R. pacifica there was a relation between the variables: large specimen size (ambitus 45-69 mm) tended to have ratio values from 0.45-0.50, the angles were between 50 and 70° (more acute than C. caribaearum) and the peristome was tall and triangular. These two patterns were noticeable in other clusters of morphological test variations. In terms of the general test morphology of C. caribaearum, the ambitus and the bulge were slightly ovoid and elongated, and dorsoventrally flattened, with low ambitus sizes between 21 and 78.91 mm. We observed within the different groups and sizes low, intermediate and high ratios between the MAW and PIW, in combination with the lowest values of the distances between ambulacra, and that specimens had an ambitus of intermediate to large size. This suggests that C. caribaearum does not have specific variations between the different sizes; intermediate morphology may exist, meaning a continuous variability. Rhyncholampas pacifica, however, has a gibbous test and shows several kinds of variations; sizes (ambitus measurements) showed that the specimens with the largest perimeters of the ambitus had the lowest THl, TWl, AIL, AIIIL, angle between ambulacra I and V and low relationships between the MAW and PIW, AIL and AIIIL measurements; conversely, the medium-sized specimens had the highest values of these features and the smallest sizes of the species (ambitus 1.92-21 mm) did not have a particular relation between them.
In the case of the angles between the ambulacra I and V, it was found that C. caribaearum tended to present values that range between 59 and 79 mm (average value = 66.76 mm, S.D. = 4.0869 mm), while for R. pacifica these values oscillated between 45 and 69 mm (average value = 59.8 mm, S.D. = 5.6382). Also, C. caribaearum tended to present rounded forms of the peristome base shape, and the forms that had larger ambitus also had angles between ambulacra I and V that were much greater than the rest of the specimens in the same species (above 70°); this was independent of their relationships between THl and TWl. Meanwhile, in R. pacifica the specimens had peristome base shape that are triangular, and the specimens with higher values of ambitus tended to present THl/TWl ratio values that were concentrated between 0.45 and 0.50 mm (Fig. 7).
In R. pacifica the organisms had a size range that can exceed 50 mm in length (largest specimen: 51.6 mm) and 40 mm (largest specimen: 42.6 mm) in width; in C. caribaearum the sizes did not exceed 25 mm in length (largest specimen: 22.9 mm) and 20 mm in width (largest specimen: 19.4 mm) (Table 3, Table 4).
Table 4 K-means (Silhoutte) cluster analysis in Rhyncholampas pacifica (Dissimilar averages are marked in bold. Minimum and maximum averages are marked in black)
| Variable | S-W test P-value | Centers (Average) | Welch t-test P-value | |
| Group 1 | Group 2 | |||
| Lateral length = TWl | 0.7440 | 30.658 | 45.4996 | 3.89 × 10-12 |
| Lateral heigh = THl | 0.7038 | 14.478 | 21.0444 | 8.12 × 10-11 |
| THl/TWl ratio | 0.6036 | 0.4726 | 0.4626 | 0.118 |
| Peristome length = PL | 0.0021 | 2.4559 | 3.4030 | 1.49 × 10-5 |
| Peristome width = PW | 0.4988 | 2.9733 | 3.5485 | 0.008 |
| PW/PL ratio | 0.4730 | 1.2277 | 1.0724 | 0.041 |
| Ambulacral I length = AIL | 0.9538 | 11.709 | 18.523 | 1.54 × 10-11 |
| Ambulacral III length = AIIIL | 0.9589 | 13.082 | 20.309 | 2.58 × 10-11 |
| AIL/AIIIL ratio | 0.8015 | 0.8943 | 0.9132 | 0.254 |
| Ambulacral I-III angle | 0.0002 | 72.296 | 69.815 | 0.240 |
| Ambulacral I-V angle | 0.1038 | 62.074 | 59.741 | 0.064 |
| Maximum ambulacral width = MAW | 0.8296 | 3.2211 | 4.6459 | 1.66 × 10-9 |
| Maximum width of the outer poriferous zone of petal I = PIW | 0.9724 | 0.5644 | 0.6681 | 0.031 |
| MAW/PIW ratio | 0.0317 | 6.1316 | 7.4129 | 0.026 |
| Size of group | 27 | 27 | ||
| Within Sum of Squares | 3905.777 | 3869.790 | ||
| Between_SS / Total_SS | 39.70 % | |||
For C. caribaearum, a first cluster includes the specimens with smaller ambitus combined with some specimens with ambitus up to 59 mm in perimeter, with a considerable number of specimens showing low ratios between MAW/PIW. A particular case for this variable is presented by a second group, where the specimens reach the highest ratios between MAW/PIW in combination with the lowest values of PIW and whose specimens have ambitus of intermediate to high size, with some specimens being in the range of 59 to 91 mm in perimeter. A third group is formed by specimens in which the ratios between MAW/PIW generally have intermediate values, in combination with slightly low values for THl, TWL, AIL and MAW. The fourth group was represented by specimens with the highest average of ambulacral angles, in combination with slightly greater distance between PIW than in the other groups. The groups and average values of the analyzed measurements are shown in Fig. 8.
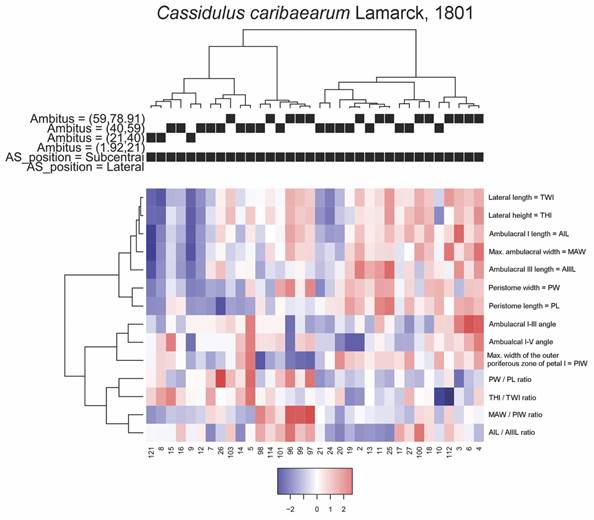
Fig. 8 Heatmap coupled to cluster diagrams for Cassidulus caribaearum. Centered variables were used for the construction of the heatmap.
Two groups were observable for R. pacifica, the first of which includes the specimens with the largest perimeters of the ambitus with generally low measurements of THl, TWl, AIL, AIIIL, and angle between ambulacra I and V, these specimens also presented weak relationships between the MAW and PIW; and AIL and AIIIL. Some of these specimens also showed slightly large values for the PIW and the relationship between THl/TWl of the organism. The second group is composed by specimens with small to medium sized ambitus, in this case the specimens of greater THl and TWl, with one of them showing even the largest sizes on the PW, another with the lowest value of the angle between I and V ambulacra and one more presenting the lowest values of PW that also corresponds to the lowest ratio between PL/PW. The groups and average values of the analyzed measurements are shown in Fig. 9.
Discussion
Cassidulus caribaearum and R. pacifica have been considered very closely related species, but Souto et al. (2019) proved that the genera Rhyncholampas and Cassidulus have been separated for more than 60 million years. However, the interspecific morphological differences between these two extant species are still being analyzed.
Souto et al. (2019) studied the intraspecific variations of the morphological features of C. caribaearum and R. pacifica, tests noticed a great variation in the number of phyllopores plates and of occluded plates in all phyllodes, and in the number of additional pore pairs in the unequal (paired) petals. Regarding test length and width (lateral view), C. caribaearum is a small to medium-sized species (neotype test measurements from Souto & Martins, 2018: total length 26.68 mm, total width 22.65 mm, and total height 11.71 mm) with oval test; the total width is approximately 85 % of the total length. The lateral edges are straight with round margins; the greatest height is at the apical disc; it has a triangular transverse section and a concave oral region (Souto & Martins, 2018). Schultz (2017) mentioned that length of C. caribaearum scarcely reaches 25 mm. In this study we found that C. caribaearum from the Mexican Caribbean Sea (50 specimens from Punta Nizuc to Punta Maroma, Quintana Roo, Mexico) does not exceed sizes of 22.9 mm length and 19.4 mm width; individual variation ranges of test length are from 3.312-22.959 mm and width are from 2.708-18.995 mm. The genus Rhyncholampas have been described as small to large of varying test shape with maximum length up to 70 mm in the living species (Agassiz, 1869; Schultz, 2017; Souto et al., 2019). This analysis showed specimens of R. pacifica from the Mexican Pacific (50 specimens from Punta Barron, Sinaloa to Acapulco, Guerrero, Mexico) clearly reach test length values of 51.5 mm and test width values of 42.6 mm; individual variation ranges of test length are from 5.01-51.59 mm and width are from 4.549-43.222 mm.
Martínez-Melo (2008) stated that test height, peristome length and distance from the peristome to the anterior edge of the test are the most significant measurements that separate C. caribaearum from R. pacifica, supporting that test dimensions are different between both species. Here we also used the test height and distance from the peristome to the anterior edge of the test, here referred as Da-pta, as variables of the ratios TH1/TWI and TLo/Da-pta which primarily contributes to the discrimination of C. caribaearum from R. pacifica in the PCA analysis. Therefore, we confirm that these measurements, as variables of such ratios, allow us to distinguish between both species. Martínez-Melo (2008) also mentioned that R. pacifica had a taller test and longer peristome than C. caribaearum. In this regard, we found test height values of 6.919 to 15.67 mm for R. pacifica, and 5.976 to 6.088 mm for C. caribaearum, agreeing with R. pacifica having a taller test. In addition, Martínez-Melo (2008) mentioned that C. caribaearum had a lower distance from the peristome to the anterior edge of the test. We confirm her statement because the Da-pta values for C. caribaearum range from 1.354 to 8.235 mm, and 1.55 to 15.789 for R. pacifica. The author explained these lower values of Da-pta for C. caribaearum as a result of the higher growth of its skeleton.
When analyzing the intraspecific variation in the test length and width in proportion, we found that R. pacifica had 1:1.047, which means it is slightly longer than it is wide; in C. caribaearum the proportion is 1:1.185, also being longer than wide. From the measurements of TWl, THl, AL, PL, PW, AIL, AIIIL, MAW, PIW, PB, AFFP, TLa/TW, TLa/Da-ppa, TLo/Da-pta, THl/TLl, PpL/PpW, A, PBS, PS, ASP, PL/PW, AIL/AIIIL, and MAW/PIW, we conclude that the small specimens developed a more inflated form during their juvenile stage, with longer and wider ambulacra; then, when they reach the adult form, they developed a more flat test gibbosity inside their own triangular, inflated shape, unlike C. caribaearum which keep almost the same shape during their transition from small to large size (3.313-22.959 mm).
Regarding the variations of the peristome, although in both species the relationship between the measurements of the top of the test and the shape of the base of the peristome is not very clear, it is possible to identify the variation of the base of the peristome between species; in C. caribaearum, the smaller specimens (lateral height and lateral width) have a straight shape, while in larger specimens the base is rounded. We observed a similar behavior in R. pacifica, since in the smaller specimens the base of the peristome is triangular and tall; on the other hand, in the larger specimens the base is perceived as completely triangular. Souto and Martins (2018) assigned a neotype of C. caribaearum (with measurements of test length = 26.68 mm and test height = 22.65 mm) mentioning that the shape of the peristome is pentagonal; this probably indicates that the growth of the test is related with the change in the shape of the peristome base and, consequently, of the complete shape of the peristome.
Rhyncholampas pacifica shows a test with slender form, which corresponds to a longer and pointier interambulacral basicoronal plate V towards the peristome while in C. caribaearum the test shape is broader with a flattened plate. As suggested by Saitoh and Kanazawa (2012), slender forms tend to dig deeper into the substratum whereas robust forms dig shallow; the anterior suggests that R. pacifica is more adapted to dig deeper in certain types of substratum (e.g. sandy) than C. caribaearum.
We confirm that R. pacifica has a taller test than C. caribaearum, while the latter has a lower distance from the peristome to the anterior edge of the test. We also recognize two intraspecific patterns between the ambulacra length and angles, and the peristome shape, for each living species.
In sum, it is demonstrated that morphometric data of the tests and peristome are useful to address taxonomical issues on recent cassiduloids, suggesting that more morphometric studies including other species could be carried out. Moreover, new morphometric analysis of the species studied here adding specimens from the rest of their geographic distribution range would be useful to compare these results.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio