Introduction
New World cutaneous leishmaniasis (NW-CL) is the most common form of leishmaniasis in Latin America with an annual average of 56 000 cases of cutaneous and mucosal leishmaniasis. It is produced by an intracellular protozoan parasite belonging to subgenus Viannia or Leishmania, transmitted to humans and reservoirs by infected female sand-flies of the genus Lutzomyia (Pan American Health Organization, 2018). Symptomatic NW-CL is characterized by skin lesions, such as papules, nodules, or ulcerative volcano-like lesions, with varied outcomes, in terms of lesion number and size, scar type, disability, spontaneous healing, and destructive mucosal inflammation (Bailey et al., 2017).
Pentavalent antimonials (SbV, Glucantime, and Pentostam) remain the standard treatment for all forms of leishmaniasis in some countries (Burza, et al.,2018). They are administered parentally in 10-30 doses of 20 mg/kg/day, depending on the clinical manifestations. Mild to severe side effects, including arthralgia, myalgia, cardiotoxicity, nephropathy, and pain during intramuscular administration, are observed (Burza et al., 2018; Goto & Lindoso, 2010). In some regions endemic for visceral leishmaniasis, such as Bihar, in Eastern India, therapeutic failure (TF) can be observed in up to 65 % of cases, while estimates of antimonial TF rates on NW-CL range from 15-32 % (Castro, et al.,2017; Sundar et al., 2000). In cases with simple CL (single or a few lesions, up to 900 mm2), pregnant women, and patients with kidney, heart disease, or other concomitant diseases, local/topical treatments, such as cryotherapy, localized heat, intralesional drugs, or topical application of semisolid formulations directly onto the lesion are alternative therapies (Burza et al., 2018).
Intralesional (IL)-SbV treatment is widely used in treatment of Old World-CL, and is applied alone, or in combination with cryotherapy. Further, intralesional (IL)-SbV therapy is recommended for simple NW-CL cases, where lesions do not involve the face or joints (PAHO, 2018). Intralesional-SbV injection is easily administered by trained staff, does not require hospitalization, can be given at a low dose, and results in less systemic drug absorption, side effects, and treatment costs; 1-5 sessions of intradermal IL-SbV injections, with 1-5 ml per session, at the border or base of the CL-lesion every 3-7 days are recommended (PAHO, 2018). The overall clinical efficacy of IL-SbV using an injectable Glucantime solution (the same one used for intramuscular route treatment) is reported as 75 to 77 % (de Oliveira Duque et al., 2019; Oliveira-Neto et al., 1997). In Bolivian patients with CL, three or five sessions of 2-3 ml each (650 μg SbV per mm2), every other day resulted in cure rates from 57 to 73 % at 6 months (Soto et al., 2016). Overall TF was 30 % (like parenteral SbV) and no recurrences were recorded in cured patients for ≥ 6 months (de Oliveira Duque et al., 2019; Oliveira-Neto et al., 1997). There is not a validated IL-SbV protocol using doses calculated as mg/kg body weight and administered over a large number of IL-sessions, with small injection volumes in humans. In addition, there is not an IL-SbV protocol available as a positive control for NW-CL intralesional drug discovery able to demonstrate both efficacy and some SbV-IL risks such as lesion reactivation and TF on experimental models.
The present study aim was to determine the efficacy of different concentrations of IL-SbV (Glucantime) administered in 29 daily sessions of 100 μL each, on mice infected with two common NW-CL species, L. (V.) panamensis and L. (V.) braziliensis.
Materials and methods
Drugs and reagents: Meglumine antimoniate, Glucantime® (Sanofi-Aventis, Brazil; lot 357345) was kindly donated by the Secretary of Health of Santander, Colombia. One bottle of Glucantime contained 1.5 g of meglumine antimoniate (405 mg of SbV). Schneider’s insect medium, RPMI medium, and heat inactivated FBS (hiFBS) were from Gibco (Grand Island, NY, USA).
Parasites and cells: Promastigotes of L. (V.) panamensis (MHOM/PA/71/LS94) and L. (V.) braziliensis (MHOM/BR/75/M2903) were maintained in Schneider’s medium plus 10 % hiFBS at 28 °C. Human leukaemia THP-1 cells (ATCC, USA) were cultured in RPMI 1640 medium plus 10 % hiFBS at 37 °C in a 5 % CO2 /95 % air mixture. THP-1 cells were transformed to adherent cells using 40 ng/ml phorbol 12-myristate 13-acetate for 48 h, and these macrophages infected with stationary-phase promastigotes at a cell: parasite ratio of 1:5 for 24 h at 32 °C. The percentage of infection was determined microscopically by Giemsa staining. Axenic amastigotes were obtained from late-phase promastigotes after 8 days of transformation by progressive changes of temperature (from 27 to 32 °C) and pH (from pH 7.0 to 5.5). The Leishmania species used were susceptible to miltefosine, pentamidine and ketoconazole (Neira, et al., 2019a).
Animals, ethics: Female and male BALB/c mice (10-12 weeks of age) were supplied by the National Health Institute (Bogotá, Colombia). Mice were housed with a 12 h light/dark cycle, at 23 °C, 55 % ± 5 % relative humidity, with access to water and food pellets ad libitum. Studies were approved by the UIS-Ethics Committee (CIENCI, Code 17-2017). Animals were anesthetized by intraperitoneal injection of a ketamine/xylazine cocktail and euthanized by cervical dislocation.
Anti-leishmanial activity in vivo: Mice were infected by subcutaneous injection in the shaven rump with 5 × 105 stationary-phase parasites. When lesion size (LS) was in the range 40-42 mm2 (8 weeks after infection), mice were randomly allocated into four groups (N = 6 per group); different concentrations of SbV (150, 50, and 16.6 mg SbV/kg body weight /day) and vehicle (0.9 % saline solution) (100 µL) were injected intralesionally (IL) for 29 days. Doses of SbV were calculated for mice using mean body weight (27 g), containing 4.1, 1.4, and 0.4 mg SbV per mouse and a total of 117.5, 39.2, and 13.9 mg SbV/mouse over 29 days. Lesion size was measured weekly using a digital calliper and lesion area (mm2) calculated (Neira et al., 2019a). Follow-up period was determined in a preliminary experiment (Appendix 1). Briefly, Leishmania infected mice (N = 1 per species) were treated with IL-SbV-150 mg/kg/day × 29 days and sacrificed 15, 22, 29, and 42-days post-treatment. The chosen follow-up time was 60 days’ post treatment (pt.).
At the end of the experiment, animals were sacrificed, and smears (imprints) prepared from lesions fixed in methanol and stained with Giemsa for detection and calculation of parasites by microscopy. Additionally, lesion samples were collected and processed for histopathological examination. Amastigote number per microscopic field (400×) was semi-quantitatively scored [No parasites = 0, scarce = 1-5 (+), moderate = 6-10 (++), abundant > 11 (+++) parasites)] and percentage LS reduction (LSred) calculated (Neira, et al.,2019b). Aesthetic efficacy (eE) was calculated as (N mice with > 75 % of LSred /6 mice) × 100, and final efficacy (fE) as (N mice with both 100 % of LSred and no parasites/6) × 100. Reactivation was defined as the appearance of a new lesion in a previously aesthetically 100 % healed site. Mean effective dose (ED)50 (dose of SbV able to reduce LS by 50 %) with 95 % confidence interval (95 % CI) was calculated by sigmoidal regression using MsxlfitTM software.
Adverse effects: Mice weight was measured weekly using a digital balance. Skin irritation was registered by visual inspection and signs of irritation at application sites classified from 0 = no irritation to 4 = severe irritation.
Histopathological analysis: Samples were fixed with 10 % neutral formalin, embedded in paraffin, and sectioned into 5 µm thick sections using a microtome. Dewaxed slices were stained with haematoxylin-eosin and examined by microscopy. Histopathological parameters and parasites were semi-quantified and scored as follows: -, absent; +, mild; ++, moderate; and +++, severe (Neira et al., 2019b).
In vitro assay: Promastigotes and axenic amastigotes were incubated with serial 1:3 dilutions of Glucantime (33.3 to 900 µg/ml) for 72 h at 27 and 32 °C, respectively (Gupta et al., 2001). Control cells were incubated in culture medium. Drug activities were assessed using a resazurin colorimetric test. Absorbance values were measured using a Synergy H1 microplate reader at 570 and 600 nm. For intracellular parasites, infected THP-1 cells were treated with Glucantime for 5 days, at 32 °C (Neira et al., 2019a). Parasite growth inhibition was determined by counting infected and non-infected cells on Giemsa-stained slides by microscopy. IC50 values were calculated as described for ED50.
Statistical analysis: Differences were analyzed using the Student’s t-test. P values ≤ 0.05 were considered statistically significant. Area under the curve values were calculated for comparisons of SbV dose-response effects using GraphPad Prism software, version 6.0 for Windows and two-way ANOVA and Sidak post-hoc method.
Results
Efficacy of treatment with IL-SbV-150 mg/kg × 29: For L. (V.) panamensis infection, eE of 83.3 % (5/6 mice; M1-M4, M6) and LSred values of 87.0-100 % were observed both at the end of treatment and 15-days pt. At 60-days pt., LS reactivation (Re) was observed in mouse M6 (Table 1, Fig. 1). Therapeutic failure was also observed in mouse M5failure. At the end of treatment and until 43-day pt., M5failure showed LSred of almost 50 %; however, a subsequent increase of LS by almost 8 times was observed (Table 1). In conclusion, 29 doses of IL-SbV-150 mg induced a fE value of 66.6 % in L. (V.) panamensis-infected mice.
Table 1 Efficacy of 29-doses of IL-SbV at 150 mg/kg/day in mice infected with L. (V.) panamensis
| Mice | Before mm2 | 29-doses-IL-SbV-150 mg on L. (V.) panamensis infected mice | ||||||||||||
| end treatment | 15-day pt. | 60-day pt. | ||||||||||||
| mm2 | LSred (%) | eE (%) | mm2 | LSred (%) | eE (%) | mm2 | LSred (%) | eE (%) | Parasites | fE | ||||
| imprints | biopsies | |||||||||||||
| M1 | 36.6 | 3.5 | 90.5 | 83.3 | 0 | 100 | 83.3 | 0 | 100 | 66.6 | - | - | 66.6 | |
| M2 | 35.4 | 0 | 100 | 0 | 100 | 0 | 100 | - | - | |||||
| M3 | 32.5 | 0 | 100 | 0 | 100 | 0 | 100 | - | - | |||||
| M4 | 45.9 | 6.2 | 86.6 | 0 | 100 | 0 | 100 | - | - | |||||
| M5 | 50.3 | 35.2 | 29.9 | 19.6 | 61.0 | 153.2 | 0 | +++ | +++ | |||||
| M6 | 46.7 | 3.1 | 93.3 | 0 | 100 | 31.6 | Reb | +++ | +++ | |||||
| SS | 13.8 | 28.7 | 0 | NDc | 30.1 | 0 | ND | 46.5 | 0 | ND | +++ | +++ | ND | |
The table shows details of individual lesion size (LS) before and at the end of treatment and at 15 and 60 days post treatment (pt.) (N = 6). Parasite loads were scored at the end of treatment. LSred: lesion size reduction; eE: aesthetic efficacy; %eE: percentage of mice with LSred between 75-100 %); fE, final efficacy: with a complete aesthetic and parasite response; SS: saline solution (a representative control mouse); Re: reactivation; ND: not determined.
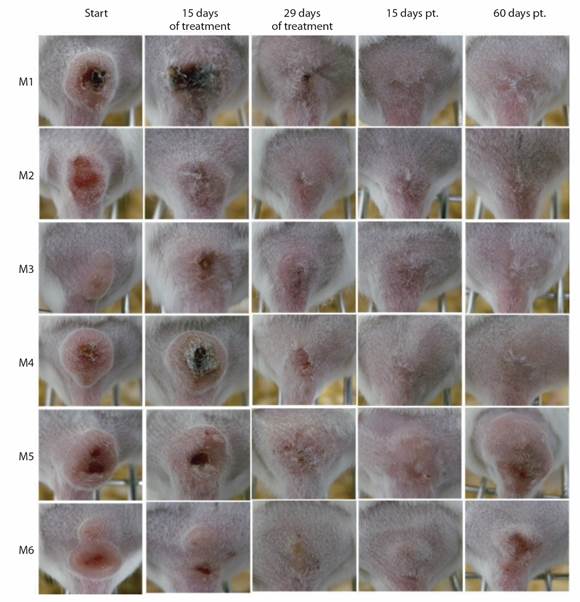
Fig. 1 Response of mice infected with L. (V.) panamensis to IL-SbV-150 mg/kg/day x 29. Photographs of CL lesions in mice M1-M6 at the beginning, 15 days and at the end of treatment (29 days); and at 15 and 60 days pt.
For L. (V.) braziliensis infected mice, an eE value of 83.3 % (5/6 mice, M1, M2, M4, M5, M6) and LSred from 80.9 to 100 % were observed both at the end of treatment and at 15-days pt. At 50-day pt., lesion reactivation was detected in mice M4, M5, and M6. Additionally, mouse M2, an aesthetically cured animal, tested positive for parasites in biopsy samples (Table 2, Fig. 2). Mouse M3 showed a slight decrease in LS at 15 days pt. and a complete aesthetic response, without observable parasites, at the end of the experiment. Consequently, fE was 33.3 %.
Table 2 Efficacy of 29 doses of IL-SbV at 150 mg/kg in mice infected with L. (V.) braziliensis
| Mice | Before (mm2) | 29-doses-IL-SbV-150 mg on L. (V.) braziliensis infected mice | |||||||||||
| end of treatment | 15-day pt. | 60-day pt. | |||||||||||
| mm2 | LSred (%) | eE (%) | mm2 | LSred (%) | eE (%) | mm2 | LSred (%) | eE (%) | Parasites | fE | |||
| Im prints | biopsies | ||||||||||||
| M1 | 55.9 | 0 | 100 | 83.3 | 0 | 100 | 83.3 | 0 | 100 | 50 | - | - | 33.3 |
| M2 | 34.9 | 0 | 100 | 0 | 100 | 0 | 100 | - | ++ | ||||
| M3 | 14.5 | 23.2 | 0 | 10.4 | 28.6 | 0 | 100 | - | - | ||||
| M4 | 32.8 | 6.28 | 80.9 | 0 | 100 | 30.6 | Reb | +++ | +++ | ||||
| M5 | 35.7 | 0 | 100 | 0 | 100 | 11.9 | Re | +++ | +++ | ||||
| M6 | 43.1 | 0 | 100 | 0 | 100 | 8.7 | Re | +++ | +++ | ||||
| SSa | 13.65 | 49.64 | 0 | NDc | 57.8 | 0 | ND | 113.5 | 0 | ND | +++ | +++ | ND |
The table shows details of individual lesion size (LS) before and at the end of treatment and at 15 and 60 days’ post treatment (pt.) (N = 6). Parasite loads were scored at the end of treatment. LSred: lesion size reduction; eE: aesthetic efficacy; %eE: percentage of mice with LSred between 75-100 %); fE, final efficacy: with a complete aesthetic and parasite response; SS: saline solution (a representative control mouse); Re: reactivation; ND: not determined.
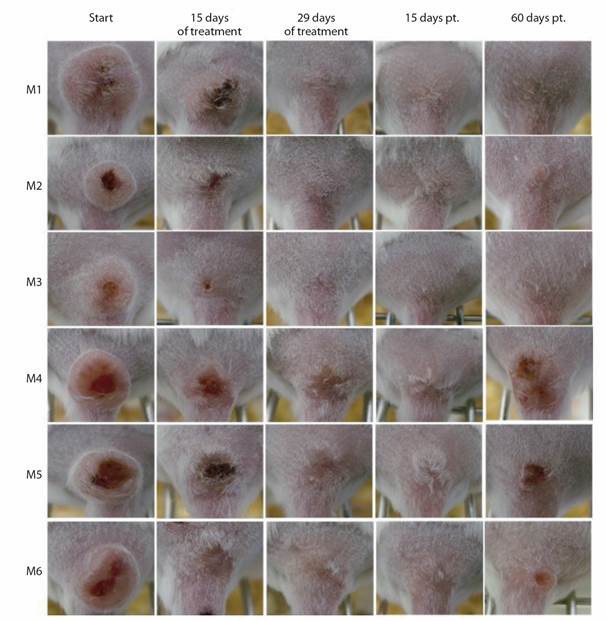
Fig. 2 L. (V.) braziliensis -infected mice response to IL-SbV-150 mg/kg/day x 29. Photographs of CL lesions in mice M1-M6 at the beginning, 15 days and at the end of treatment (29 days); and at 15 and 60 days pt.
All non-cured mice showed abundant (+++) intra and extracellular amastigotes in CL-lesion samples.
None of the mice treated with 29 doses of IL-SbV showed body weight loss, skin irritation, or any signs of pain or loss of well-being.
Efficacy of lower doses (IL-SbV-50 mg/kg x 29 days and IL-SbV-16.6 mg/kg x 29 days): At 15-day pt., L. (V.) panamensis infected mice M4, M5, and M6 treated with IL-SbV-50 mg, displayed considerable improvement (LSred 89.5-100 %) (Appendix 2, Appendix 3) and stable effects were also observed in mice M1, M2, and M3 (LSred 43.7-58.4 %); however, at 60-day pt., reactivation or increase of LS was observed. In mice treated with IL-SbV-16.6 mg, LSred values from 0 to 51.6 % were observed both at the end of treatment and 15 days pt.; however, at the end of the experiment, fE was zero and abundant (+++) parasites were observed by microscopy in imprint smears and biopsies (Appendix 2, Appendix 4).
For L. (V.) braziliensis infected mice, IL-SbV-50mg treatment induced LSred. of 80-100 % in mice M2 and M4-M6 at 15 days pt.; however, reactivation occurred in two of these mice (M2 and M6) and there was an increase of LS in another (M4) with parasites present in all of them, including M5 (Appendix 5, Appendix 6). Treatment with IL-SbV-16.6mg induced a LSred of 87.2 % in one mouse (M6) at 15 days pt.; however, TF occurred at 60-day pt. Parasites were observed in lesions from all treated mice by microscopy (Appendix 5, Appendix 7).
Cutaneous leishmaniasis lesions after relapse (Re): Mouse M6Re treated with IL-SbV-150 mg and mice M4Re and M6Re treated with IL-SbV-50 mg after infection with L. (V.) panamensis had new lesions of LS of 31.6, 11.6, and 3.5 mm2, respectively, at the end of the experiment. Mice M4Re-M6Re, treated with IL-SbV-50 mg and M2Re and M6Re, with IL-SbV-16.6 mg, following L. (V.) braziliensis infection, relapsed with new lesions of LS 30.6, 11.9, 8.6, 7.8, and 20.0 mm2, respectively, at the end of the experiment. Lesions reappeared at the inoculation site and began as papules, that gradually evolved into nodules with raised edges. In some cases, they presented as typical clean, pink ulcers, with granular tissue, and rounded with regular and raised edges.
Dose response activities of SbV: The mean effective dose (ED)50 values for L. (V.) panamensis and L. (V.) braziliensis infected mice were 33.99 (CI, 18.89-61.14) mg and 36.96 (CI, 17.87-76.45) mg at the end of treatment; 28.62 (CI, 16.53-49.55) mg and 12.40 (CI, 4.41-34.91) mg at 15 days pt.; and 142.6 (CI, 57.56-353.3) mg and 157.8 (CI, 58.99-421.9) mg at 60 pt., respectively. At 43 days pt., IL-SbV was more active in mice infected with L. (V.) braziliensis than L. (V.) panamensis (P < 0.001); however, no difference was observed at the end of the experiment (60 days pt.). No significant differences were detected in the response to IL-SbV between mice infected with the two species of Leishmania based on analysis of LS or area under the curve values for lesion changes after treatment with at 150, 50, or 16.6 mg/kg/day IL-SbV × 29.
Histopathological characteristics: Responders (cured) mice (M1-M4 for L. (V.) panamensis; M1 and M3 for L. (V.) braziliensis) showed none to mild changes in the epidermis and dermis (Appendix 8, Appendix 9, Appendix 10). In contrast, M5failed or M6Re for L. (V.) panamensis, M4Re-M6Re for L. (V.) braziliensis, and negative control mice presented with severe inflammatory infiltrates, comprising lymphocytes, neutrophils, macrophages, and giant cells. Some mice presented with severe acanthosis and moderate spongiosis and necrosis (Appendix 8).
In vitro drug activity: Based on IC50 values, SbV was inactive against all live forms of parasites evaluated, since IC50 values were > 900 µgSbV/ml for both Leishmania strains.
Discussion
In this work, we evaluated the responses of CL-infected mice to Glucantime, administered over a large number of IL-sessions, with small injection volumes each day (e.g., not until lesions blanched) and SbV regimens calculated by body weight (kg), instead of LS (mm2). Administration of the highest dose of IL-SbV (equivalent to 98.78 µg SbV/mm2 × 29 daily, and 2 864.6 µg SbV/mm2 in total) was effective in 4/6 and 2/6 mice infected with L. (V.) panamensis and L. (V.) braziliensis at 60 days pt.
Some positive results were obtained with IL-SbV in a hamster CL model (100 % lesion reduction and no parasites in organs) (Travi, et al., 1993; Yépez, et al.,1999); however, no complete cure of CL-infected mice has been documented. Per example, treatment of mice infected with L. (L.) amazonensis, with IL-SbV 28 mg/kg/day × 5, every 4-5 days resulted in partial parasite burden reduction with no increase in footpad thickness, but not in cure (Cos et al., 2018; Fournet et al., 1996). Differences in numbers of SbV-IL sessions, dose, site of infection, parasite species, and follow-up time could explain the different results obtained in this study. In addition, type of CL lesion could be also involved; ulcerated lesions (Fig. 1, Fig. 2) without parasite dissemination or spontaneous cure are characteristic of our CL-mouse model (Neira, et al., 2019b). The final SbV doses/per mouse (at a body weight, 27 g) also differed. At 150, 50, and 16.6 mg/kg/day × 29, the final doses were 117.5, 39.2 and 13.9 mg SbV, while at 28 mg/kg × 5 the dose was 3.78 mg 224 SbV (almost three times less than the minimum non-effective dose used in our study).
IL-SbV treatment induced a dose-response effect; drug potency was higher soon after treatment than at the end of experiment, as ED50 values were lower at 15 than 60 days pt. Hence, the maximal SbV concentration used may have been unable to kill 100 % of parasites on all treated mice, with remaining parasites able to reactivate lesions, as was observed in some mice at the end of the experiment. In a previous study we reported the histopathological characteristics of CL lesions before treatment (Neira et al., 2019b). They were characterized moderate hyperkeratosis and acanthosis, mild spongiosis in the epidermis, with a severe diffuse inflammatory infiltrate predominantly comprised of lymphocytes and plasma cells, and abundant amastigote-infected macrophages in the dermis. It is possible that a higher mgSbV/kg dose will be necessary to kill all parasites.
Next, we decided to evaluate SbV activity in vitro on promastigotes, axenic amastigotes, and intracellular amastigotes of both Leishmania strains infecting THP-1 cells, using the same batch as that used for in vivo experiments. Unfortunately, we were unable to calculate IC50 values (drug potency) at the maximal doses evaluated in any of the live parasite forms of either parasite species. The antileishmanial activities of SbV have been demonstrated in various in vitro models, showing some peculiarities compared with other drugs such as miltefosine or pentamidine isethionate (Fernández et al., 2014). Most notably, SbV is unable to kill promastigotes and, to a lesser extent, axenic amastigotes (Sereno et al., 1998); however, activities have been demonstrated against intracellular amastigotes infecting different types of macrophage and reference strains or isolates collected worldwide (IC50 2.9-146 µgSbV/ml) (Fernández et al., 2014; Pérez-Franco et al., 2016). In general, drug susceptibility (in vitro) is supposed as a method to predict treatment outcome. However, this was not our case as SbV was active on some CL infected mice but not on free or intracellular parasites life forms. Both reference strains used are not classified as Sb-resistant strains. In contrast, SbV activity has been demonstrated against L. (V.) braziliensis /M2903 intracellular amastigotes infecting U937 differentiated cells (IC50 26-50 µgSbV/ml) (Pérez-Franco et al., 2016). Before our experiments, the parasites had never been exposed to antimonials in neither in vitro or in vivo assays; however, they have been cultivated in vitro for a relatively extended period, by quarterly passage in BALB/mice. Leishmania resistance mechanisms or other factors related to drug (drug quality, intrinsic drug properties, drug physicochemical characteristics), parasite (lower intrinsic susceptibility to the drug, parasite infection by RNA viruses, parasite adaptations, manipulation skills of the parasites, higher parasite fitness) or host (immunological factors, pharmacokinetics, genetics) could be involved with SbV treatment efficacy or failure (Vanaerschot et al. 2014).
Independent of the lack of in vitro SbV response, the use of intralesional SbV with a dose calculated as mg/kg body weight and administered over a large number of IL-sessions, with small injection volumes each day could be effective against L. (V.) panamensis and L. (V.) braziliensis-CL infection. An appropriate SbV-dose (higher than 150 mg/kg/day x 20) must be evaluated.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.
Digital Appendix











 uBio
uBio 


