Introduction
Eastern Tropical Pacific (ETP) coral reefs are small, dominated by few species, and with a discontinuous distribution, presenting a typical zonation pattern where shallow zones are mostly dominated by ramose monogeneric communities of Pocillopora spp., and massive species and leafy taxa, such as Porites spp. and Pavona spp., increases in dominance with depth (Toth et al., 2017), whose persistence depends mainly on the connectivity through larval dispersal between demes (Fobert et al., 2019; Sale et al., 2005). This connectivity determines gene flow, coral community structure and metapopulation dynamics (Ayre & Hughes, 2000). The extent of connectivity by self-recruitment or long-distance immigration, is strongly associated to their resilience against natural and anthropogenic disturbances (Almany et al., 2007; Underwood et al., 2009). The main ETP’s disturbances that cause bleaching events and local to regional mass mortalities are: seasonal sea surface temperatures (SST) with El Niño and La Niña events (Glynn et al., 2017); solar irradiance (LaJeunesse et al., 2010); hurricanes (Aranceta-Garza et al., 2012); fisheries and tourism related impacts (Cortés & Reyes-Bonilla, 2017). The recovery of reefs has been documented as a slow process in ETP (Guzmán & Cortés, 2007), usually depending on recruitment from distant surviving colonies.
The aim of ‘no-take’ in marine protected areas (MPA) is to mitigate the anthropogenic impacts by conserving biodiversity and preventing overfishing, thereby acting as source populations to seed demographic sinks 47 within larval dispersal range (DOF, 2014; Ley General del Equilibrio Ecológico y la Protección al Ambiente, 2021; Munguia-Vega et al., 2018). As such, the connectivity within MPA networks is a fundamental process that promotes metapopulation resilience (Grimsditch & Salm, 2006). The Gulf of California (GC) has seven multi-use marine protected areas (MUMPAs) directly protecting the main reef builder, Pocillopora verrucosa, which represents a protected marine area of 3.64 % of the GC (CONANP, 2020). The effectiveness of these MUMPAs on enhancing the conservation prospects of P. verrucosa remains unknown. Nonetheless, this network of MUMPAs may integrate a regional network, as has been previously shown to be an effective managing tool elsewhere, for the conservation of biodiversity, biological processes, and critical habitats in developing countries (Munguia-Vega et al., 2018).
The distribution of P. verrucosa is delimited by the ETP ranges from GC to Ecuador (Pérez-Vivar et al., 2006). In the southern GC, they are found in ~400 km of the peninsular coast including nearby islands (Reyes-Bonilla et al., 2005). This species is a simultaneous hermaphrodite with mixed modes of reproduction (Campos-Vázquez, 2014). They are a sexual broadcast spawner with a reproductive period from June to September (Campos-Vázquez, 2014) which should confer long dispersal potential (Chávez-Romo & Reyes-Bonilla, 2007) and also recruit locally by asexual fragmentation (Aranceta-Garza et al., 2012; Pinzón et al., 2012). While few studies have addressed regional connectivity in Pocillopora species along the Mexican Pacific (MP), Chávez-Romo et al. (2008) found restricted connectivity between GC and Gulf of Tehuantepec regions, a pattern also seen in other coral species (brooder Porites panamensis: Paz-García et al., 2012b; broadcast spawner Pavona gigantea: Saavedra-Sotelo et al., 2011). Nevertheless, some of these studies suggest an unrestricted gene flow in Pocillopora type 1 species (as P. verrucosa is categorized, Pinzón et al., 2012) throughout the ETP. Previous Pocillopora connectivity studies specifically in the GC have explored few locations, ranging from 10 to 100 km (less than ~30 % of its peninsular range). They have found high connectivity between demes (Chávez-Romo, 2014; Chávez-Romo et al., 2008; Paz-García et al., 2012b; Pinzón et al., 2012), but with contrasting local genotypic structures (asexual vs sexual recruits), showing that demes are strongly influenced by local factors (Aranceta-Garza et al., 2012; Pinzón et al., 2012). This variation in the contribution of sexual and asexual reproductive strategies have important consequences for Pocillopora resilience and disturbance recovery, with recent studies suggesting that environmental conditions in the GC favored coral sexual reproduction (Cabral-Tena et al., 2018; Chávez-Romo, 2014).
Until recently, most connectivity studies in the GC have use low resolution molecular markers (Delmotte et al., 2002; Ridgway & Gates, 2006), such as allozyme or internal transcribed spacer (e.g.: Chávez-Romo et al., 2008; Paz-García et al., 2012b; Saavedra-Sotelo et al., 2011). Polymorphic microsatellites can detect fine-scale connectivity by shifting from populations to individuals at relevant ecological scales (Gélin et al., 2018; Taninaka et al., 2019). To date, only two studies have explored the connectivity of P. verrucosa populations in the GC using microsatellite markers (Pinzón & LaJeunesse, 2011; Pinzón et al., 2012), however, their sampling locations examined were few and geographically close.
Here we assess the connectivity patterns of P. verrucosa in the Southern GC, by applying assignment test methods, where population genetic statistics assign individuals to the putative natal population (Piry et al., 2004). These assignment methods have been applied in other corals species, to show the spatial scales of connectivity, source-sink dynamics and population resilience capabilities (e.g. Seriatopora hystrix in Maier et al., 2009; Acropora austere and Platygyra daedalea in Montoya-Maya et al., 2016; Heliopora spp. in Taninaka et al., 2019).
We hypothesize that P. verrucosa conforms a highly connected metapopulation in the GC and its dispersal capabilities are related with regional oceanographic features characterized by a series of gyres that reverse direction twice a year (Marinone, 2012); yet each deme population structure will also be influenced by local factors (e.g. hurricanes, bleaching). We also hypothesize that due to the environmental seasonal characteristics, intermediate range MUMPAS will protect source demes with a crucial role for regional coral recovery. As such, the research objectives were (a) to assess genetic diversity and variation in P. verrucosa using microsatellites; b) to assess the population structure in the GC; c) to estimate larval migration patterns and dispersal distances between demes along the GC; d) to determine the role and effectiveness of preestablished MUMPAS in Pocillopora protection and resilience; and e) to explore implications of the estimated connectivity, as means of dispersal direction and magnitude, for the effective management of hermatypic corals in the GC.
Materials and methods
Study area: The study area of P. verrucosa encompasses its northernmost limit in North America along the peninsular coast of the Gulf of California (GC) (Fig. 1), where it grows either as a coral reef (as in Cabo Pulmo, Bahía San Gabriel in Isla Espíritu Santo, and San Lorenzo Channel), or more commonly as patch reefs (El Portugués and Loreto). Its populations are subjected to seasonal environmental factors, such as high irradiance, cold water upwellings, hurricanes, and oceanographic currents. The seasonal marine circulation in the GC is dominated by temporal forcing agents, which results in contrasting seasonal periods: spring and summer; and fall and winter (Marinone, 2003). During the spring and summer, the Western half of the Southern Gulf of California (SGC) has a surface circulation dominated by a cyclonic gyre directed southwardly and creating a dominant peninsular northerly coastal current. During fall and winter, this gyre reverses with dominant southwardly coastal current. The SGC gyre current velocities run at 0.25-0.50 ms-1 (Lavín et al., 2014).
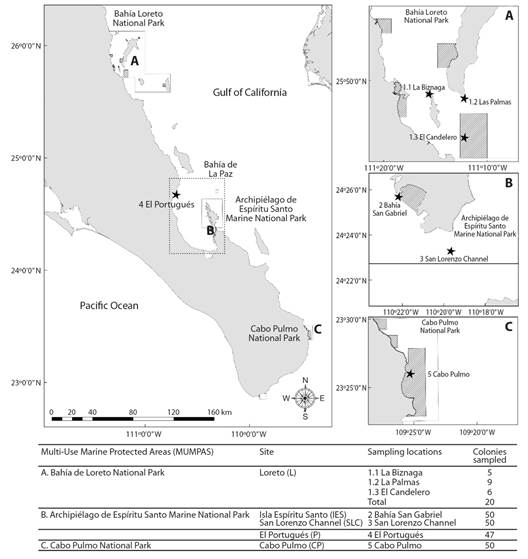
Fig. 1 Sampling sites (black stars) of Pocillopora verrucosa in the peninsular coast of the Gulf of California. Solid black lines show multi-use marine protected area (MUMPA) polygons. Diagonal line fill areas show MUMPAs ‘no take’ zones.
Sample collection: Coral species identification is complex and can be based on morphological characters, molecular identification or both (Budd et al., 2012; Toller et al., 2001) For the GC, Pinzón and Lajeunesse (2011), using the mitochondrial open reading frame (mtORF), found that the morphotypes P. verrucosa, P. damicornis, P. meandrina, P. capitata and P. eydouxi belong to a single lineage defined as type 1, however, Flot et al. (2008) mentioned the existence of ambiguities in the molecular identification these species. Therefore, the present study considers that all the colonies sampled should be regarded according to Pinzón and Lajeunesse (2011) as mtORF type 1. However, the identification of the nominal species of P. verrucosa was based on morphological diagnostic characters (Veron, 2000) conducted at the Laboratorio Virtual de Sistemas Arrecifales (LAVISA) at the Universidad Autónoma de Baja California Sur by regional coral expert Dr. Hector Reyes.
For the sampling design each colony was established as an independent experimental unit. Samples of Pocillopora verrucosa colonies (Fig. 1) were collected in the SGC from February 2008 to June 2009. The colonies were selected randomly avoiding neighboring heads (2 m minimal distance among colonies) at a maximum depth of 7 m and were collected by fragment removal (~2 cm3). Sampling locations from North to South were: (a) an exposed reef (i.e. intense wave and current energy) in Bahía Loreto National Park (L); an exposed reef in El Portugués (P); Archipelago de Espíritu Santo Marine National Park with two locations: a protected bay in Isla Espíritu Santo (IES) and an exposed reef in San Lorenzo Channel (SLC); and an exposed reef in Cabo Pulmo National Park (CP). L samples were collected at three areas: Las Palmas at Carmen Island, La Biznaga at Danzante Island, and Islet El Candelero. The three areas were taken as a single location (denoted by “L”; N = 20) since they did not show significant allelic frequency differences previously tested by an analogous Fisher’s exact test (Guo & Thompson, 1992); (b) Bahía de La Paz area samples included those from the peninsular location at P (N = 47 colonies) and IES at Bahía San Gabriel (N = 50 colonies) and SLC coral reefs (N = 50 colonies); (c) the southernmost sampling location was in CP reef (N = 50 colonies).
DNA extraction and microsatellite genotyping: The fragments were preserved in 70 % ethanol and stored at 4 °C, pending DNA extraction. Total genomic DNA from samples was extracted using a commercial kit (DNEasy kit, Qiagen, Valencia, CA) per manufacturer’s instructions. The quality of the extraction was verified by agarose gel electrophoresis (Sigma, St. Louis, MO). Each DNA extraction was quantified using a low DNA mass ladder (Invitrogen, Life Technologies, Carlsbad, CA) in 1 % agarose gel electrophoresis.
A polymerase chain reaction (PCR) was carried out using six microsatellites developed for Pocillopora spp.: PV6 and PV7 (Magalon et al., 2004), and Pd3-002, Pd3-005, Pd2-006, and Pd3-008 (Starger et al., 2008). PCR amplifications of each microsatellite were performed in a thermocycler (Applied Biosystems, Life Technologies, Carlsbad, CA) with a final volume of 12.5 µL containing 50 to 100 ng DNA template, 1 × buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 0.5 µM of each primer, and 0.625 units µL-1 polymerase (Platinum Taq polymerase, Invitrogen). The thermal cycling program was: 10 min at 94 °C + 30 × (45 s at 94 °C + 45 s at 55 °C + 30 s at 72 °C) and + 8 min at 72 °C. PCR products were visualized on an acrylamide gel using a multi-view scanner (FMBIO III, Hitashi, Yokohama, JP). Re-visualizations were made to confirm allele sizes. The resulting bands were scored manually using 10 bp standard ladders (#10821015, Invitrogen). Only two loci showed stuttered bands (PV6 and Pd2-006), obscuring the allele reading, which were correctly reamplified using the cycling protocol of Yoshida et al. (2005):1 × (2 min at 94 °C ), 5 × (5 s at 94 °C, 60 s at 56 °C, 60 s at 72 °C), 20 × (0 s at 94 °C, 60 s at 56 °C, 60 s at 72 °C), and 1 × (60 min at 72 °C).
Genotyping: Genotypes were analyzed using GIMLET (Valière, 2002) to identify individuals with same multi-locus genotypes (MLG). GIMLET also estimates the probability of identity (PID), that is, the probability that two individuals in the population are identified as clone mates when in fact they are distinct genets. The theoretical expected PID and the unbiased PID were computed for each locus using allele frequencies from the genotype scores (see Waits et al. (2001) for the formulation). According to Waits et al. (2001), PID scores ranging from 0.001 to 0.0001 should be sufficiently low for forensic applications in natural populations, giving a very low probability of misidentifying clone mates in this study.
Two datasets were then constructed for subsequent genetic analyses: (1) full MLG (i.e. including clonal individuals) and (2) unique MLGs.
Identification and correction of null alleles for all loci was made using the program MICRO-CHECKER (Oosterhout et al., 2004). Genetic variability was obtained per location based on: mean allelic richness (A); effective allele number (ne); observed (H o ); and expected (H e ) heterozygosities, which were obtained using the program Arlequin 3.11 (Excoffier et al., 2005).
Microsatellite data were checked for departures from Hardy Weinberg Equilibrium (HWE) and linkage equilibrium (Arlequin v.3.11) adjusting significance values with Bonferroni corrections (Rice, 1989).
Population genetic structure was assessed using the pairwise F-statistics (Arlequin v.3.11) with 10 000 permutations and α = 0.05.
Migration patterns: To assess P. verrucosa isolation by distance patterns, we tested for statistical significance between geographical and genetical distance (F ST / (1 - F ST )) matrices using the program IBDWS Version 3.23 (Jensen et al., 2005). Significance tests (P < 0.05) were obtained with a Mantel test using 10 000 randomizations. Contemporary migration patterns were assessed using Geneclass 2.0 (Piry et al., 2004), which computes genetic assignment criteria to include/exclude reference populations as the origin to diploid individuals. The five sampled populations were analyzed using the six microsatellite loci of the unique MLG dataset representing sexual planula connectivity. Briefly, the analysis employed a Bayesian assignment criterion (Rannala & Mountain, 1997) with a Monte Carlo resampling (Paetkau et al., 2004) of 100 000 iterations, generating the probability of likelihood to include/exclude a colony (or individual) to a sampled population.
We used two approaches to estimate individual migration. First, the detection of immigrants in the sampling populations (i.e. first-generation migrants) was assessed using the likelihood of individual genotypes within the population where the individual had been sampled (L_home). An assignment threshold α = 0.05 was used to accept the alternative hypothesis that the individual was an immigrant. Second, the possible origin of each detected immigrants among the sampled populations was evaluated using an assignment criterion with α = 0.10, where the population with the highest probability was selected as the most likely source population. When immigrants resulted in a P-value < 0.1 the software assumed they belonged to other unsampled populations.
Results
A total of 217 P. verrucosa colonies were sampled along 350 km in the Gulf of California. Only 99 (45.6 %) of these had unique MLG. The rest (N = 118,54.4 % of the sampled colonies) were clones of one of the unique MLGs. Clonal individuals were observed at all the sampling sites and ranged from ∼40 % of the sampled individuals in Cabo Pulmo, El Portugués and Loreto to ∼95 % in San Lorenzo Channel and Isla Espíritu Santo. No MLG were share among localities.
The probability of identity (PID) for the full MLG was 4.12 × 10-05 (biased approach) and 3.67 × 10-05 (unbiased approach); the results for the unique MLG was 2.00 × 10-05 (biased approach) and 1.50 × 10-05 (unbiased approach).
Genetic diversity: The allelic diversity (A) for the full MLG dataset ranged from 4-10 alleles with a maximal mean of 6.33 alleles ( 2.58 SE, N = 6) in Cabo Pulmo and minimal of 4.00 alleles ( 0.89 SE, N = 6) in San Lorenzo Channel (Table 1).
Table 1 Pocillopora verrucosa allele richness (A), effective alleles (ne) and observed (Ho) and expected (He) heterozygosity per locus in the five populations sampled in the Gulf of California using the unique multi-locus genotypes
| Unique Multilocus Genotypes (UMLG) | ||||||||
| CP | SLC | IES | P | L | ||||
| N | 37 | 6 | 5 | 35 | 16 | |||
| PV6 | A | 10 | 5 | 6 | 10 | 5 | ||
| ne | 6.86 | 3.13 | 4.17 | 6.43 | 3.91 | |||
| Ho | 0.92 | 0.67 | 1.00 | 0.77 | 0.88 | |||
| He | 0.87 | 0.74 | 0.84 | 0.86 | 0.77 | |||
| PV7 | A | 8 | 5 | 5 | 8 | 6 | ||
| ne | 3.22 | 2.57 | 3.85 | 3.04 | 3.76 | |||
| Ho | 0.73 | 0.67 | 1.00 | 0.71 | 0.69 | |||
| He | 0.70 | 0.67 | 0.82 | 0.68 | 0.76 | |||
| Pd3-005 | A | 7 | 4 | 4 | 5 | 5 | ||
| ne | 1.87 | 2.06 | 2.38 | 1.79 | 2.18 | |||
| Ho | 0.51 | 0.50 | 0.60 | 0.43 | 0.69 | |||
| He | 0.47 | 0.56 | 0.64 | 0.45 | 0.56 | |||
| Pd3-002 | A | 6 | 3 | 4 | 5 | 4 | ||
| ne | 2.24 | 1.95 | 2.94 | 2.77 | 3.66 | |||
| Ho | 0.51 | 0.67 | 0.80 | 0.49 | 0.31* | |||
| He | 0.56 | 0.53 | 0.73 | 0.65 | 0.75* | |||
| Pd3-008 | A | 4 | 4 | 3 | 4 | 4 | ||
| ne | 1.49 | 2.06 | 1.85 | 1.94 | 1.48 | |||
| Ho | 0.32 | 0.50 | 0.40 | 0.46 | 0.31 | |||
| He | 0.33 | 0.56 | 0.51 | 0.49 | 0.33 | |||
| Pd2-006 | A | 3 | 3 | 3 | 5 | 4 | ||
| ne | 2.95 | 2.88 | 2.94 | 3.27 | 2.93 | |||
| Ho | 0.70 | 1.00 | 0.80 | 0.74 | 0.75 | |||
| He | 0.67 | 0.71 | 0.73 | 0.70 | 0.68 | |||
| A ± SD | 6.3 ± 2.58 | 4.0 ± 0.89 | 4.2 ± 1.17 | 6.2 ± 2.32 | 4.7 ± 0.82 | |||
| ne ± SD | 3.1 ± 1.95 | 2.4 ± 0.49 | 3 ± 0.87 | 3.2 ± 1.69 | 3.0 ± 0.98 | |||
| Ho ± SD | 0.62 ± 0.21 | 0.67 ± 0.18 | 0.77 ± 0.23 | 0.6 ± 0.16 | 0.6 ± 0.24 | |||
| HE ± SD | 0.6 ± 0.19 | 0.63 ± 0.09 | 0.71 ± 0.12 | 0.64 ± 0.15 | 0.64 ± 0.17 | |||
*Significant at P < 0.05 for Hardy-Weinberg departure using a sequential Bonferroni correction; CP: Cabo Pulmo; SLC: San Lorenzo Channel; IES: Isla Espíritu Santo; P: El Portugués; L: Loreto.
Linkage disequilibrium was not statistically significant for the unique MLG dataset. Null alleles were observed in the locus Pd3-002 (P < 0.001) for Loreto resulting in an excess of homozygotes.
The tests of HWE assumptions was analyzed in the unique MLG dataset (i.e. no clone individuals) all loci were in equilibrium, except for the locus (Pd3-200) in Loreto with an heterozygote excess. Cabo Pulmo was the only location in HWE in both datasets.
Genetic Structure: Pairwise F ST analyses (Table 2) were contrasting between full and unique MLG datasets where the F-statistics over all loci were statistically significant for the former with a F ST = 0.108 *** and non-significant for the latter with a F ST = 0.0007 NS. Pairwise F ST analysis in the unique MLG showed only one significant genetic differentiation between population extremes, Loreto and Cabo Pulmo (F ST = 0.021*). This observation was also supported by a significant Mantel test (P < 0.05) using the unique MLG dataset.
Table 2 Pairwise multi-locus estimates of F ST between populations of Pocillopora verrucosa in the Gulf of California. Populations labelled from South to North
| Unique Multi-locus Genotype (UMLG) | |||||
| CP | SLC | IES | P | L | |
| CP | - | ||||
| SLC | -0.017 | - | |||
| IES | -0.012 | -0.028 | - | ||
| P | 0.001 | -0.013 | -0.029 | - | |
| L | 0.021* | -0.010 | -0.017 | 0.011 | - |
Significant * at P < 0.05; ** at P < 0.01; *** at P < 0.001; CP: Cabo Pulmo; SLC: San Lorenzo Channel; IES: Isla Espíritu Santo; P: El Portugués; L: Loreto.
Contemporary migration patterns: The ecological time scale migration patterns suggested 10 individuals identified as putative immigrants between sampling locations (P < 0.05, Table 3). All the locations showed immigrants: Cabo Pulmo had the most (N = 3), Isla Espíritu Santo had the least (N = 1) (Table 3, see letter A, Fig. 2). Three unassigned immigrants (two in El Portugués and one in Cabo Pulmo) originated in an unsampled locality. The mean distance range traveled by the seven planulae was of 116.6 km (± 80.5 SE) (Table 3, see letter B). The spacing between the Archipiélago de Espíritu Santo Marine National Park with respect to Bahía Loreto and Cabo Pulmo National Parks was 180 km and 165 km, respectively, exceeding the mean planulae dispersal distance in 64 and 49 km, accordingly (Table 3, see letter B).
Table 3 Number of individuals (immigrants) excluded from their sampling site and their most probably deme of origin (assigned deme) (A) and observed dispersal distance matrix and multi-use marine protected area (MUMPA) interspace (B)
| (A) | Assigned deme1 | Immigrants (n) | Immigrants unassigned (n) | ||||
| Sampling deme | L | P | SLC | IES | CP | ||
| L | * | 2 | 2 | ||||
| P | * | 2 | 2 | ||||
| SLC | 1 | * | 1 | 2 | |||
| IES | 1 | * | 1 | ||||
| CP | 1 | 1 | * | 3 | 1 | ||
| Total | 0 | 3 | 0 | 4 | 0 | 10 | 3 |
| (B) | Distance matriz (km) | MUMPAs | Interspace (km) | ||||
| Sampling deme | L | P | SLC | IES | CP | ||
| L | * | 180 | AES→CPNP | 165 | |||
| AES→LBNP | 180 | ||||||
| P | * | ||||||
| SLC | 49 | * | 7 | ||||
| IES | 42 | * | |||||
| CP | 200 | 158 | * | ||||
| Mean dispersal distance | (116.571 ± 80.473) | Mean MUMPA interspace | (172.5 ± 10.60) | ||||
1Individuals were excluded from their sampling site at a P-value < 0.05; individuals were assigned to their putative deme of origin with the highest probability of occurrence; excluded individuals with P-value < 0.1 were assumed to have originated from a deme not sampled in the study. L: Loreto; P: El Portugués; SLC: San Lorenzo Channel; IES: Isla Espíritu Santo; CP: Cabo Pulmo; AES = Archipiélago de Espíritu Santo Marine National Park; LBNP = Bahía de Loreto National Park; CPNP = Cabo Pulmo National Park.
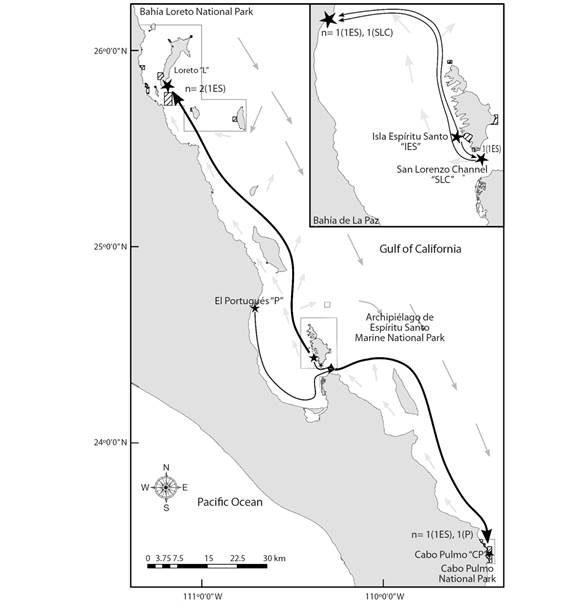
Fig. 2 Connectivity patterns of the seven assigned immigrants (n) for the Pocillopora verrucosa planulae in the Gulf of California. Black stars show the demes; black solid arrow shows the planula dispersal direction, where brackets indicate the immigrant source deme. Light and heavy gray solid arrows show the summer coastal and oceanic current direction, respectively; they inverse during the fall-winter seasons (in Marinone, 2003).
Discussion
This study examines for the first time, the ecological connectivity patterns of the main coral reef builder in the Gulf of California, Pocillopora verrucosa. At a local scale, P. verrucosa deme maintenance showed a mixed mode reproductive strategy. Some locations almost exclusively made of clones (Isla Espíritu Santo and San Lorenzo Channel); some showed intermediate levels of clonality and sexual recruitment (El Portugués and Loreto), while others were dominated by sexual planula recruitment (Cabo Pulmo). On a regional scale, all the demes were in panmixia, acting as a metapopulation with differential migrant contributions allowing to differentiate between source and sink demes. However, significant genetic differentiation was observed between the most distant demes, Loreto and Cabo Pulmo National Parks. This emphasizes the importance of the mid-range Bahía de la Paz area (i.e. Archipiélago de Espíritu Santo National Park and El Portugués deme), which appears to act as a stepping stone for connecting distant sink demes and maintaining metapopulation resilience.
Genetic diversity and structure: The genetic diversity found in the GC (A = 5.07 ± 0.68) was similar to other studies of subtropical populations of Pocillopora: Africa: A = 5.0 ± 1.4 in Ridgway et al. (2008); Australia: A = 6.3 ± 3.23 in Thomas et al. (2014); and Panamá A = 7 ± 2.4 in Combosch and Vollmer (2011), and contrasts with the higher diversity seen at low latitudes: Polynesia A = 9 ± 5.2 in Magalon et al. (2004). This difference may reflect the effects of isolation and low effective population size on genetic diversity (Ayre & Hughes, 2004).
The heterogenous frequency of clones presented in all the locations emphasize the important role of asexual reproduction for local maintenance (e.g. by storm fragmentation). This suggests a coral’s adaptive strategy towards local factors where a few specialized clonal genotypes redirected resources towards repair and growth (Aranceta-Garza et al., 2012). Recently, this process could have been mechanically enhanced by documented anthropogenic impacts, such as shipwrecks and recreational activities such as anchoring, scuba diving and also fishing nets (Aranceta-Garza et al., 2012; López-Espinosa de los Monteros, 2002). The observed frequencies of sexual colonies over the locations was explained previously in Aranceta et al. (2012), suggesting a relation with external factors such as type of substrate and coral density, where higher hard substrata and less coral density could be promoting sexual recruitment (e.g. Cabo Pulmo, Loreto and El Portugés).
The connectivity by sexual migrants between demes showed a P. verrucosa metapopulation in panmixia on the peninsular GC. However, extreme demes such as Loreto and Cabo Pulmo, showed low levels of connectivity (Table 2), suggesting that most of the dispersal occurs between neighboring demes and long-distance events may be rare. Moreover, Loreto is the Northern limit of P. verrucosa’s ETP range and often experiences severe disturbances, which may explain the few and isolated standing colonies surrounded by dead coral heads. This results in a sink population, dependent from Southern larvae, showing low genetic diversity whilst observing and excess in heterozygote (Balloux, 2004). Contrary to local asexual maintenance, sexual larvae dispersal is an important process for coral resilience in GC (Chávez-Romo, 2014), ensuring demes replenishment and recovery in areas impacted by natural or anthropogenic factors (McLeod et al., 2009).
Pocillopora verrucosa contemporary connectivity: The assignment test results suggested that most larvae dispersing out of their region (70 %) originated in the Bahía de La Paz area, mainly from both Archipiélago de Espiritu Santo National Park and the unprotected deme El Portugués. However, there was no long-distance dispersal between extreme demes (~330 km between Cabo Pulmo and Loreto), which could be attributed to physical factors such as oceanographic GC retention features: gyres, fronts, bays and islands. Moreover, mean dispersal distance was 116.6 km (± 80.5 SE) and the observed source-sink dynamics revealed that Bahía de La Paz is a key source area, supplying larvae to North (Loreto) and South sink demes (Cabo Pulmo) and depend on the seasonal direction of oceanographic currents and gyres (Marinone, 2003) during P. verrucosa reproductive period. These results further demonstrate the importance of conserving central demes for coral replenishment and recovery, enhancing regional metapopulation resilience. One recent example was Loreto, presenting 90 % loss of coral cover which has been slowly recolonized by Southern larvae (Hernández et al. 2010; LaJeunesse et al., 2010; Paz-García et al., 2012a), probably from Bahía de La Paz. Furthermore, these newly recruited individuals probably will be genetically and physiologically more adapted (i.e. thermal tolerant zooxanthellas) to conditions of very high/low temperature and light intensity (Iglesias-Prieto et al., 2004; LaJeunesse et al., 2007; Paz-García et al., 2012a; Reyes-Bonilla, 2001). This adaptive process may have already been demonstrated after the 2015 ENSO disturbance, where no Pocillopora colony mortality was reported in Loreto (Reyes-Bonilla, personal communication, 2021).
Management implications: The incorporation of connectivity among demes in the P. verrucosa metapopulation, including the existing MUMPA network in the Southern GC, combines with other considerations for their conservation prospects. The coral reefs are fragile ecosystems whose resilience depends not only on their biology, but on other species, especially herbivorous fish and invertebrates (Grimsditch & Salm, 2006). Current management schemes in the GC-MUMPAs permit fishing activities over large areas, leaving just a few small areas as ‘no-take’ zones, reducing the MUMPA conservation value by decreasing fish species, functional richness, and their ecosystems processes over time (Ramirez-Ortiz et al., 2020), affecting reef resilience. If the expansion of these ‘no-take’ is not possible, another recommended conservation tool for the protection of coral reefs in the GC is the establishment of critical habitats, as defined in the General Wildlife Law (Ley General de Vida Silvestre, 2018).
The mean dispersal distance in P. verrucosa (116.6 km) was longer than the individual length of the multi-used marine protected areas, but shorter than mean spacing between installed MUMPAs (172.5 km). Given these estimates, we suggest the designation of two new ‘no-take’ zones to be integrated with existing Southern GC-MUMPAs. This management measure would reduce the separation between Archipiélago Espíritu Santo and Cabo Pulmo (to the South) and Loreto (to the North) MUMPAs to ~50 km each, which agrees with the minimum spacing recommended for coral connectivity (50-200 km in Munguia-Vega et al. (2018), although 10-20 km in McLeod et al. (2009). Moreover, the resulting MUMPA network would also cover a variety of temperature regimes (tropical and subtropical) to increase the survival rate in bleaching events (Iglesias-Prieto et al., 2004). Finally, including the El Portugués source deme under a ‘no-take’ regimen would build up ecological redundancy into the network against large scale disturbances (Munguia-Vega et al., 2018). All the above measures will promote the preservation of regional coral connectivity, mainly by stepping-stone recolonization events coming from central and South demes, which are valuable for short term recovery in continuous reef systems (Underwood et al., 2009) and may apply for GC reefs and patch systems.
The genetic assignment methods we applied here contributed to the identification of the dispersal patterns and level of connectivity in the P. verrucosa metapopulation within an installed MUMPA network in the GC. This study determined that the central peninsular area functions as a key source of larvae to other locations, promoting metapopulation resilience by enhancing recovery of damaged reefs with Southern thermal tolerant larvae, especially to higher latitude areas. Moreover, anthropogenic disturbances should be removed from coral habitats by changing current management schemes to ‘no-take’ zones to protect connectivity, reproduction and ecosystem functional diversity. The inclusion of intermediate ‘no-take’ zones in the MUMPA network, as well as adding redundant source areas (El Portugués), would provide a stepping-stone refuge and habitat replication in case of severe damaged. This study provides as a baseline for policymakers and authorities to establish robust strategies for coral ecosystem protection and to promote metapopulation resilience from natural and anthropogenic factors.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


