Introduction
Interspecific interactions are essential to determine species richness and composition in natural communities (Chase & Leibold, 2003; Colorado-Zuluaga, 2015; Diamond, 1975). In particular, competition is considered a critical interaction since its reduction or avoidance promotes resource partitioning of ecologically similar species (Holt, 2001; Klatt et al., 2015). Thus, temporal and spatial segregation of ecologically similar species can result from the effect of interspecific competition, which may have ecological and evolutionary implications by constituting a mechanism that allows coexistence between species and, so, affecting the structure of biological communities (Kronfeld-Schor & Dayan, 2003). The removal of an ecologically similar species leads to changes in the temporal activity patterns and habitat use of competing species (Ziv et al., 1993), suggesting temporal segregation; however, it is considered less common than dietary specialization and spatial segregation (Schoener, 1974). Consequently, the ecological significance of segregation in temporal activity to facilitate the coexistence of species is still controversial (Zeppelini et al., 2017), and this segregation is usually associated with the circadian rhythms of species (Kronfeld-Schor & Dayan, 2003).
The temporal activity patterns of species can vary across their geographic ranges in response to biotic and abiotic factors (Vieira et al., 2017). In small mammals, the degree of flexibility in the temporal activity pattern is associated with predation risk, so species with a higher predation risk tend to have less flexible activity patterns (Halle & Stenseth, 2000). Besides, abiotic factors (such as temperature, precipitation, and photoperiod) tend to influence the activity patterns of certain groups of organisms (Kronfeld-Schor & Dayan, 2003; Vieira et al., 2017), with different effects and magnitudes depending on the animal group (Vieira et al., 2017). Variations in the daily activity patterns of species between locations possibly reflect fluctuations in food availability or foraging strategies (O’Donnell, 2010; Vieira et al., 2017). Due to the multiple factors that can influence the temporal activity of species, assessing the importance of temporal segregation as a mechanism of coexistence requires understanding spatial and temporal variation in activity patterns of ecologically diverse groups. However, partitioning of time among species has been less studied than spatial partitioning.
Bats are highly diverse, abundant, and ecologically important in tropical forests; thus, understanding their activity patterns can help unravel the mechanisms that determine the local diversity of assemblages. Temporal activity patterns are known for some species of Neotropical bats (Brown, 1968; Rocha et al., 2020; Zeppelini et al., 2017), vary spatially, and are influenced by the availability of resources: vegetation structure, nectar availability, energy requirements, and activity patterns of their preys (Kunz, 1973; Rothenwöhrer et al., 2011). For insectivorous bats, the temporal variation in activity patterns is related to the temporal distribution of insects (Hagen & Sabo, 2014; Kuenzi & Morrison, 2003; Speakman et al., 2000). For canopy frugivores, there are regulatory factors of the activity patterns as climatic seasons and lunar cycles, mainly associated with full moon nights (Lang et al., 2005; Santos-Moreno et al., 2010; Zeppelini et al., 2019) and several unpredictable factors such as precipitation and environmental disturbance (López-González et al., 2012; Milne et al., 2005). However, despite the high local richness of bats, time partitioning as a mechanism structuring bat assemblages remains poorly studied.
Neotropical frugivorous bats, in particular, are a rich local guild with a high potential for niche overlap and interspecific competition, resulting from a close phylogenetic relationship since all species belong to the Phyllostomidae family. Thus, studying Neotropical frugivorous bats’ activity patterns can help to understand the mechanisms that allow the coexistence of species in highly diverse assemblages. For bats of Carolliinae subfamily, differences in nocturnal activity among closely related have been reported (Bonaccorso et al., 2007; Delaval et al., 2005). Besides, base on a high spatial overlap on foraging sites, the differences in mean emergence time among Carollia castanea y C. brevicauda seem to avoid competition and suggests the existence of exploitative competition between them (Bonaccorso et al., 2007). However, comparisons of activity patterns between pairs of closely related species have concluded that the activity peaks of fruit bats have not evolved to reduce competition between them (Aguiar & Marinho-Filho, 2004; Zeppelini et al., 2017). Hence, empirical studies are needed to fully understand the importance of segregation in temporal activity as a mechanism that, by reducing competition, promotes coexistence in bat assemblages.
To understand the importance of time partitioning for the coexistence of ecologically similar species, we studied the temporal activity patterns of assemblages of Neotropical frugivorous bats in three ecosystems (tropical dry forest, wetforest, and rainforest). We compared the degree of overlap in temporal activity at different levels: A) Community, contrasting assemblages between study areas; B) inter-specific, comparing species inside each of the assemblages; and C) intra-specific, comparing the same species between study areas. We compared temporal activity by estimating the degree of overlap in hourly activity between species pairs. Additionally, we evaluated the association between the degree of overlap in temporal activity and the differences in body size, abundance, and phylogenetic relationship at each site. We predict that: (1) the temporal overlap between assemblages of bats will be low, indicating that temporal activity patterns will be different between assemblages due to adjusting of species to contrasting local environmental and biotic conditions between sites (O’Donnell, 2010; Vieira et al., 2017; Vilella et al., 2020); (2) in each site, the overlap in temporal activity of species will decrease as species are more similar in size, abundance, or phylogenetically (Castro-Arellano & Lacher, 2009; Nagy-Reis et al., 2018) and (3) the temporal overlap between populations of the same species will be low, indicating that temporal activity patterns of the same species will differ between sites due to adjusting of species to contrasting local biotic and abiotic conditions between sites (O’Donnell, 2010; Vieira et al., 2017; Vilella et al., 2020).
Materials and methods
Study area: The research was carried out in three study sites that, according to Holdridge (1967), represent different life zones in Valle del Cauca, Colombia (Fig. 1). 1) El Vínculo Regional Natural Park (3°50’12” N & 76°17’58” W): A protected fragment of tropical dry forest of 83 ha (Funagua, 2010) with wooded areas in different stages of natural regeneration such as intervened primary forest (Bp-i), secondary forest (Bs), and scrub (M) (Cadelo-Cabrera & Parra-Valencia, 2007). El Vínculo has altitudes between 997 and 1 150 m above sea level (m.a.s.l.), an average temperature of 25 °C, and an annual rainfall of 1 380 mm. 2) Pericos Natural Reserve (3°50’39” N & 76°47’22” W): A community-managed reserve that contains an extensive area of wet forest (440 ha) in the Choco Biogeographic region, on the Western slope of the Western mountain range of the Andes. The reserve is located in the Dagua river basin at 500 m.a.s.l., has a high annual rainfall of above 5 000 mm, and temperatures above 24 °C (Funagua, 2010). 3) Bachué Nature Reserve (3°19’50” N & 76°39’15” W): A private reserve of 2 000 ha of rainforest with intervened primary and secondary forest areas in the Tropical Andes region. It is located in the Farallones de Cali National Natural Park buffer zone at 1 800 m.a.s.l., at the confluence of the Pato and Pance rivers. The temperature varies from 16 to 24 °C and has an annual rainfall between 2 000 and 3 000 mm (Fundación Farallones, 2013).
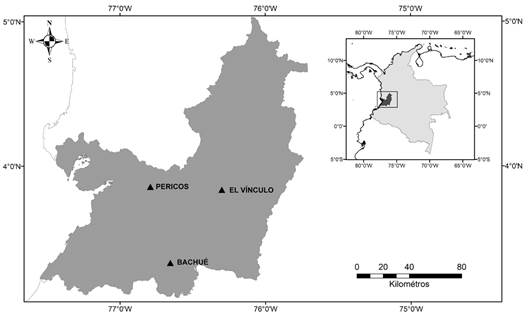
Fig. 1 Study area showing sampling sites. Dry forest (El Vínculo Regional Park), wet forest (Pericos Community Reserve), and rainforest (Bachué Reserve).
Sampling method: We sampled bats between July and October 2018 using mist nets. Each study site was sampled once at nighttime for a total sampling of 44 nights: 15 nights at the Vínculo and Pericos and 14 nights at Bachué. To determine the activity patterns of the frugivorous bats, we captured specimens using ten mist-nets (2.5 × 12 m) for a total of 158 400 m2-net/h in the localities: 54 000 m2-net/h in Vínculo and Pericos, and 50 400 m2-net/h in Bachué.
The mist nets were deployed in areas suitable for bat captures, such as forest clearings, trails, and water sources. We relocated them every two nights to include the variation in vegetation cover of each locality. The nets were checked hourly, from 18:00 to 6:00 h, due to the low catch rates of individuals. However, in case of rain, intervals of net checking were shorter, or nets were closed. We kept all captured individuals in cloth bags and classified them taxonomically using a field guide from South America (Díaz et al., 2016). Additionally, the weight of each individual was recorded and considered as a surrogate of body size. To avoid pseudo-replication, individuals were marked with haircuts in the lower dorsal region (close to the uropatagium). Finally, we fed individuals with fruit compote before being released. The study was covered by the permit for the sampling and handling of wild animals 1 070 (28/08/2015) issue by National Authority for Environmental Licenses (ANLA) to Universidad del Valle. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Data analysis: We used the distribution that describes the probability of capture within any particular hourly interval of the day to characterize the temporal activity patterns. Thus, we used the probability density function, calculated with the Kernel method of the overlap R-package (Meredith & Ridout, 2017), to describe activity patterns. First, we calculated the activity pattern for each assemblage of frugivorous bats (by adding all the capture data from a given site) and for each species at each locality. For analysis, we only considered species with at least ten records. Posteriorly, we quantified the extent of overlap between the correspondent estimated distributions of two assemblages or species to compare activity patterns. The overlapping coefficient (Δ1) quantifies the area of overlap between pairs of density curves and is recommended for small sample sizes (less than 50 records per species) (Meredith & Ridout, 2014). So, we used Δ1 to quantify the differences in hourly activity between assemblages or species. This coefficient varies between 0 (no overlap) and 1 (complete overlap) (Linkie & Ridout, 2011; Meredith & Ridout, 2017). Finally, we generated 95 % confidence intervals for ∆1 using the Bootstrap method from 1 000 repetitions (Meredith & Ridout, 2014). Then, we used these confidence intervals to determine whether activity patterns were different (between assemblages and populations of species in various sites) or whether temporal segregation was present (between species at each site). Between sympatric species of genus Carollia, the early emergence of 14 min for the small species (C. castanea) is enough to foraging, avoiding competition with the more dominant and larger species (C. brevicauda) (Bonaccorso et al., 2007). Thus, we considered differences or temporal segregation when the upper levels of confidence intervals for the overlap index were less than 0.9, indicating that the activity patterns diverged by at least 10 %.
To determine whether the overlap in the activity patterns was related to the ecological similarity of species, we evaluated the association between Δ1 and similarity in abundances and body mass and phylogenetic closeness. For this, we calculated matrices of similarity in activity patterns (Δ1), differences in body mass and abundance, and phylogenetic distance for each pair of species at each site. We calculated the abundance matrix based on the differences in captures between species from the same locality, the body size matrix based on the difference in average body mass between each pair of species, and the phylogenetic matrix based on the patristic distances (index of phylogenetic distance) between species pairs using a phylogeny of Noctilionoidea superfamily (Rojas et al., 2016), which includes more than 90 % of genera and 76 % of the superfamily species. Finally, to determine the degree of association of the temporal overlap matrix with the matrices of abundance, body size, and phylogenetics, we performed Mantel tests using the vegan package (Oksanen et al., 2017) in the R programming language (R Core Team, 2017).
Results
A total of 21 species (350 individuals) of frugivorous bats were captured. The highest species richness was found in the site of wet forest (Pericos: 13 species), followed by the site of rainforest (Bachué: 10 species), and the site of dry forest (El Vínculo: 7 species). Frugivorous bats of the genera Carollia and Artibeus obtained the highest number of records in the wet forest and dry forest sites, while those of genus Sturnira were dominant in the rainforest site. Small frugivorous bats, such as those of genus Vampyressa, had a low number of captures (Table 1).
Table 1 Species richness and abundance of frugivorous bats in El Vínculo (dry forest), Pericos (wet forest), and Bachué (rainforest) study sites
| Species (sp. / spp.) | Locality | ||
| El Vínculo | Pericos | Bachué | |
| Artibeus aequatorialis | 0 | 16 | 0 |
| Artibeus jamaicensis | 0 | 3 | 0 |
| Artibeus lituratus | 26 | 0 | 2 |
| Artibeus planirostris | 22 | 0 | 0 |
| Carollia brevicauda | 3 | 16 | 50 |
| Carollia castanea | 0 | 27 | 1 |
| Carollia perspicillata | 10 | 16 | 1 |
| Dermanura anderseni | 0 | 0 | 8 |
| Dermanura Phaeotis | 1 | 16 | 0 |
| Dermanura sp 1 | 3 | 0 | 0 |
| Dermanura sp 2 | 0 | 12 | 0 |
| Enchistenes hartii | 0 | 0 | 2 |
| Platyrrhinus chocoensis | 0 | 3 | 0 |
| Platyrrhinus dorsalis | 0 | 7 | 1 |
| Rhinophylla alethina | 0 | 21 | 0 |
| Sturnira bogotensis | 0 | 0 | 20 |
| Sturnira erythromos | 0 | 0 | 2 |
| Sturnira koopmanhilli | 0 | 1 | 0 |
| Sturnira ludovici | 0 | 0 | 41 |
| Sturnira parvidens | 0 | 0 | 2 |
| Uroderma convexum | 10 | 1 | 0 |
| Vampyressa thyone | 0 | 6 | 0 |
| Total | 75 | 145 | 135 |
The activity patterns of assemblages of frugivorous bats were variable among sites. The assemblage at the dry forest showed a bimodal pattern, with activity peaks during the first two hours after sunset and the last two hours before sunrise (Fig. 2A). On the other hand, the activity patterns of the wet forest and rainforest assemblages were unimodal, with a peak of activity during the first hours of the night and a gradual decrease in activity (Fig. 2B, Fig. 2C) respectively. The observed overlap coefficients (∆1) in hourly activity was lower between the sites with unimodal activity pattern than between them and the site with bimodal pattern: Wet forest - dry forest (∆1 = 0.675, IC = 0.555-0.751) , wet forest - rainforest (∆1 = 0.791, IC = 0.612-0.838) and dry forest - rainforest (∆1 = 0.588, IC = 0.444-0.652).
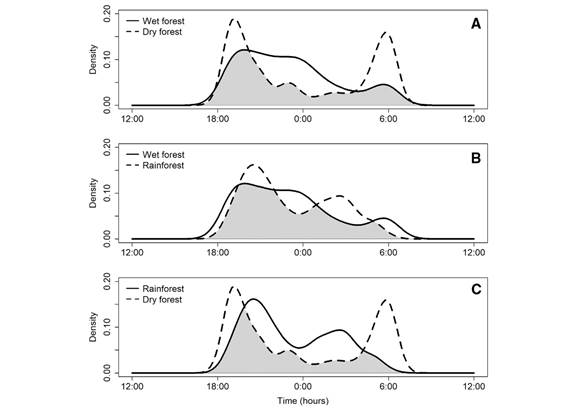
Fig. 2 Estimates of activity patterns overlap for frugivorous bat assemblages between different ecosystems: Dry forest (El Vínculo Regional Park), wet forest (Pericos Community Reserve), and rainforest (Bachué Reserve). Comparison between A. Wet forest and dry forest B. Wet forest and rainforest C. Rainforest and dry forest. The shaded area represents the overlap coefficient (∆1).
The overlap in temporal activity patterns between sympatric species of frugivorous bats (Table 2) was high and variable. At the dry forest of El Vínculo, the observed overlap coefficients (∆1) in hourly activity were on average 0.76 ± 0.06 with a difference greater than 10 % for all pairs of species (upper level of confidence intervals less than 0.9); except for Carollia perspicillata and Artibeus lituratus (Table 2).
Table 2 Mean hourly overlap (95 % confidence interval) in temporal activity between the most abundant species of frugivorous bats in a dry forest (El Vínculo Regional Park)
| Species (sp.) | Artibeus lituratus | Artibeus planirostris | Carollia perspicillata | Uroderma convexum |
| Artibeus lituratus | 1 | 0.708 (0.452-0.850) | 0.878 (0.500-0.907) | 0.748 (0.408-0.862) |
| Artibeus planirostris | 1 | 0.714 (0.366-0.851) | 0.766 (0.391-0.882) | |
| Carollia perspicillata | 1 | 0.737 (0.325-0.847) | ||
| Uroderma convexum | 1 |
At the wet forest of Pericos, the observed overlap coefficients were on average 0.70 ± 0.16, with 20 out of the 27 comparisons between species differing by more than 10 % (confidence intervals with values lower than 0.9) (Table 3). This difference was especially marked between Rhinophylla alethina and Carollia castanea since the confidence interval for ∆1 was the lowest (∆1 = 0.530, IC = 0.359-0.800) (Table 3), indicating a difference of more than 20 % in the activity patterns of these two species. This marked segregation between the activity patterns of both species is because the peak activity of R. alethina occurs around 21:00 h, when the activity of C. castanea has decreased (Fig. 3).
Table 3 Mean hourly overlap (95 % confidence interval) in temporal activity between the most abundant species of frugivorous bats in a wet forest (Pericos Community Reserve)
| Species (sp.) | Artibeus aequatorialis | Carollia brevicauda | Carollia castanea | Carollia perspicillata | Dermanura sp. 2 | Dermanura phaeotis | Platyrrhinus dorsalis | Rhinophylla allethina |
| Artibeus aequatorialis | 1 | 0.765 (0.442-0.907) | 0.684 (0.427-0.882) | 0.731 (0.400-0.879) | 0.644 (0.442-0.880) | 0.884 (0.517-0.937) | 0.654 (0.250-0.855) | 0.548 (0.313-0.813) |
| Carollia brevicauda | 1 | 0.686 (0.454-0.891) | 0.792 (0.471-0.919) | 0.713 (0.396-0.896) | 0.835 (0.477-0.938) | 0.668 (0.250-0.866) | 0.751 (0.490-0.925) | |
| Carollia castanea | 1 | 0.782 (0.545-0.922) | 0.868 (0.528-0.917) | 0.690 (0.435-0.888) | 0.680 (0.332-0.879) | 0.534 (0.359-0.800) | ||
| Carollia perspicillata | 1 | 0.833 (0.458-0.917) | 0.773 (0.438-0.899) | 0.642 (0.273-0.853) | 0.615 (0.386-0.855) | |||
| Dermanura sp. 2 | 1 | 0.677 (0.327-0.868) | 0.685 (0.327-0.868) | 0.570 (0.418-0.890) | ||||
| Dermanura phaeotis | 1 | 0.616 (0.235-0.851) | 0.604 (0.347-0.863) | |||||
| Platyrrhinus dorsalis | 1 | 0.564 (0.200-0.814) | ||||||
| Rhinophylla allethina | 1 |
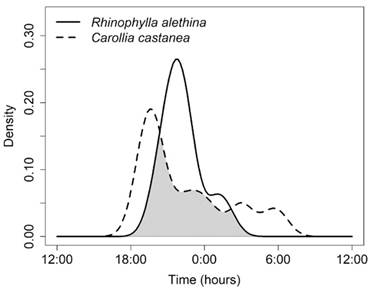
Fig. 3 Estimates of activity patterns overlap for R. alethina y C. castanea in the site of wet forest (Pericos Community Reserve). The shaded area represents the overlap coefficient (∆1).
Finally, at the rainforest of Bachué, the average overlap coefficient was 0.67 ± 0.11, with differences greater than 10 % between the two species of Sturnira (S. bogotensis and S. ludovici) and between them and C. brevicauda (Table 4). The overlap was particularly lower between S. bogotensis and S. ludovici (∆1 = 0.456, IC = 0.269-0.677). The activity of S. bogotensis is restricted to the first four hours at night (18:00-22:00 h), while S. ludovici increases after 21:00 h and remains relatively constant until before dawn (Fig. 4).
Table 4 Mean hourly overlap (95 % confidence interval) in temporal activity between the most abundant species of frugivorous bats in a rainforest (Bachué Reserve)
| Species (sp.) | Carollia brevicauda | Dermanura cf. anderseni | Sturnira bogotensis | Sturnira ludovici |
| Carollia brevicauda | 1 | 0.764 (0.474-0.930) | 0.666 (0.472-0.863) | 0.741 (0.560-0.89) |
| Dermanura cf. Anderseni | 1 | 0.694 (0.339-0.900) | 0.718 (0.354-0.915) | |
| Sturnira bogotensis | 1 | 0.456 (0.269-0.677) | ||
| Sturnira ludovici | 1 |
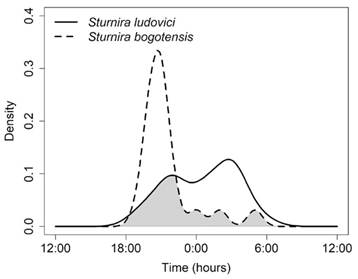
Fig. 4 Estimates of activity patterns overlap for S. bogotensis and S. Ludovici in the site of rainforest (BachuéReserve). The shaded area represents the overlap coefficient (∆1).
We found contrasting activity patterns between populations of the same species from different locations. For Carollia perspicillata, we found a difference greater than 10 % in temporal activity between Pericos and El Vínculo populations (Δ1 = 0.695, CI = 0.415-0.896), with El Vínculo individuals exhibiting a second activity peak just before the dawn that was not observed in Pericos (Fig. 5A). However, Carollia brevicauda´s activity patterns did not differ between Pericos and Bachué (Δ1 = 0.800, CI = 0.498-0.924), with both populations having higher activity at the beginning of the night and a gradual decrease after (Fig. 5B).
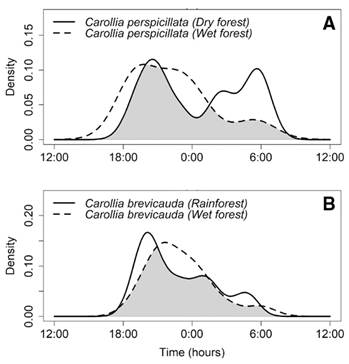
Fig. 5 Estimates of activity patterns overlap for frugivorous bats between localities. A. C. perspicillata in wet forest (Pericos Community Reserve) and dry forest (El Vínculo Regional Park). B. C. brevicauda wet forest (Pericos Community Reserve) and rainforest (BachuéReserve). The shaded area represents the overlap coefficient (∆1).
We found evidence for an association between the temporal overlap and traits related to ecological similarity in some sites with high effect sizes indicating moderate to strong correlations (R > 0.64) but no significant due to the small sample sizes involved in the tests. We found a positive and marginally significant association of temporal overlap (Δ1) with abundance (R = 0.647, P = 0.166) and body mass (R = 0.657, P = 0.083) in El Vinculo, and with phylogenetic distance in Bachué (R = 0.829, P= 0.083) (Table 5). On the other hand, we did not find associations of temporal overlap with body mass, abundance, or phylogenetic similarity in the assemblage of Pericos. Thus, these results indicate that species similar in abundance, size, and phylogenetic history tend to have less overlap in activity patterns.
Table 5 Summary of Mantel test applied to assemblages of frugivorous bats comparing effects of abundance, body mass, and phylogenetic distance into temporal segregation estimated by the hourly overlap in the temporal activity
| Locality | Abundance | Body mass | Phylogenetic distance | ||||
| R | P-value | R | P-value | R | P-value | ||
| Vínculo | 0.647 | 0.166 | 0.657 | 0.083 | 0.085 | 0.5 | |
| Pericos | -0.344 | 0.866 | -0.037 | 0.638 | -0.354 | 0.874 | |
| Bachué | -0.058 | 0.485 | 0.486 | 0.167 | 0.829 | 0.083 | |
The correlations with marginally significant associations are highlighted in bold.
Discussion
We found differences in the temporal activity pattern of frugivorous bats assemblages among sites, revealed by a difference greater than 10 % in activity patterns of assemblages. We also found differences in activity patterns between species in each locality and populations of the same species among sites. Finally, we found that species similar in abundance, size, and phylogenetic history, tend to have less overlap in activity patterns. Consequently, our results suggest that (1) local biotic and abiotic conditions may influence variation in the activity patterns between assemblages and populations of the same species, and (2) ecologically similar species tend to present less overlap in their activity patterns. Thus, our results provide empirical support for the role of segregation in temporal activity as a mechanism that allows the coexistence of ecologically similar species.
We obtained a low capture rate of phyllostomid bats that contrasts with studies in other Neotropical sites (La Val, 1970), but it is consistent with previous studies at Reserva Pericos (Zapata-Mesa et al., 2017) and El Vinculo (Velásquez-Roa & Murillo-García, 2019) and in another wet forest at similar altitude on the Western Andes of Colombia (Ferro-Muñoz et al., 2018). As we expected, the assemblages of frugivorous bats showed spatial variations in their activity patterns. Given the high energy cost and predation risk implicit in bat flight (Erkert, 1982; Norberg et al., 1993), the ecological tendency is to minimize foraging time (Howe, 1979). On the other hand, frugivores could require more time to satisfy their daily energetic requirements than other trophic guilds since their food can have a higher proportion water and indigestible materials (Erkert, 1982; Thomas, 1984). Bats spend most of the daytime in shelters, a period in which they do not feed, so they are expected to leave their shelters early in the night to solve this situation of energy stress (Aguiar et al., 2014; Ortêncio Filho et al., 2010). The frugivorous bats of the three assemblages showed the highest activity during the first hours of the night. This pattern is widely documented in neotropical bats and can result from synergistic factors (Erkert, 1982; Marinho Filho & Sazima, 1989; Pedro & Taddei, 2002; Verde et al., 2018). In this sense, the activity time, which initiates with the emergence from shelters, is also influenced by the diet of the species and the shelter’s proximity to food sources (Ortêncio Filho et al., 2010). With an activity peak early in the night, the unimodal pattern observed in Pericos and Bachué is characteristic of frugivorous species (De Souza & Marinho-filho, 2004; Erkert, 1982; Ramirez-Pulido & Armella, 1987). However, frugivorous bats at El Vínculo showed a bimodal pattern of activity, which peaks just after sunset and before sunrise. The availability of food sources can be low in El Vínculo since it is a small fragment of tropical dry forest, which could probably force frugivorous bats to perform a second feeding trip to supply their daily energy requirement. Alternatively, variations in activity between assemblages may correspond to species responses to differences between study sites in other ecological factors, such as vegetation coverage, luminosity, etc.
When there is a high overlap in one of the dimensions of the niche (for example, in the food source), a low overlap is expected in other dimensions (such as the foraging schedule) (Pianka, 1974). Experimental studies have induced temporal changes of activity in small mammals attributed to competition, resulting in a species being restricted to a less favorable activity time due to the influence of the more dominant species (Ziv et al., 1993). Likewise, previous studies have reported differences in the temporal activity patterns of frugivorous bats (Aguiar & Marinho-Filho, 2004; Zeppelini et al., 2017). Some authors suggest that this difference in the temporal activity does not result from competition (Aguiar & Marinho-Filho, 2004; Zeppelini et al., 2017), even though exploitation competition has been reported between species of Neotropical frugivorous bats (Bonaccorso et al., 2007). However, differences in activity patterns between frugivorous bats may suggest an effect of competition, with the early emergence of a species to avoiding competition during foraging with the more dominant and larger species (Bonaccorso et al., 2007).
In habitats with limited resources, it is highly probable to expect competition among bats for the fruits available a given night (Bonaccorso et al., 2007). Consequently, the bats that emerge early can successfully find food (Bonaccorso et al., 2007) since the availability of ripe fruits decreases throughout the night; consumed fruits are not renewed on the same night (Aguiar & Marinho-filho, 2004; Heithaus et al., 1975). Thus, a species can roost closer to the feeding patches or advance its emergence time to avoid competition with other ecologically similar species (Bonaccorso et al., 2007). We considered that a 10 % difference in temporal activity between two species could be enough to suggest temporal segregation. In agreement with the hypothesis of temporal segregation of resources between the species of the same guild, we found differences of at least 10 % in the activity patterns of pairs of sympatric species in the three habitats; it suggests that frugivorous bats alter their activity periods in order to reduce competition with similar species. We found marked differences between the activity patterns of species with similar body mass such as C. castanea - R. alethina in the wet forest site and between species of the same genus such as S.ludovici - S. bogotensis in the rainforest site.
Across the Neotropic region, there is a high association between genera of plants and frugivorous bats. Bats of the genus Artibeus have an affinity for the fruits of plants of the genus Cecropia, bats of Dermanura for Ficus, bats Carollia for Piper, and bats of Sturnira for Solanum (Agnarsson et al., 2011; Estrada & Fleming, 2012; Howell & Burch, 1974; Muller & Reis, 1992), with species showing some discrepancies at specific sites (Bonaccorso, 1979; Handley et al., 1991). These associations have been previously found in our study sites (El Vinculo: Velásquez-Roa & Murillo-García, 2019; Pericos: Zapata-Mesa et al., 2017), which may reduce intergeneric competition and explain the high overlap in the activity patterns we observed inside the local assemblages. Besides diet, vertical stratification in neotropical bats assemblages (Delaval et al., 2005; Pereira et al., 2010; Silva et al., 2020) may be a mechanism of spatial segregation. It can reduce the probability of encounters between bats foraging mainly in the canopy (i.e., Artibeus) and the understory (i.e., Carollia, Sturnira), reducing competition intensity. On the other hand, foraging strategies can also promote coexistence (Delaval et al., 2005; Soriano, 2000). Nomadic frugivores like species of Sternodermatinae subfamily (except Sturnira) feed on trees distant from each other by foraging over large areas tracking resource availability, whereas sedentary frugivores forage over short distances by staying in permanent roosts (Soriano, 2000). Therefore, the combination of spatial and temporal segregation with behavioral strategies may lead to coexistence and be critical to determining the structure of frugivorous bats’ assemblages, as has been reported for insectivorous species (Razgour et al., 2011).
As predicted, we found an association of temporal overlap with traits that suggest ecological similarity: abundance, body size, and phylogenetic closeness. As species differ more in body mass and phylogenetic history, they increase their temporal overlap, suggesting a tendency for ecologically similar species to segregate temporally. This effect is significant among the most abundant species, which may have a greater probability of interacting with each other (Meyer & Kalko, 2008; Patterson et al., 2003). However, we did not find these associations for all traits in all the sites studied, suggesting that the degree of ecological similarity between species may occur through different mechanisms or that the structuring mechanisms differ between sites or ecosystems. On the other hand, predation risk is a fundamental aspect considering activity patterns, particularly for small species such as bats. The segregation of activity times can reduce predation risk since bat predators can detect the aggregations of foraging bats in fruiting plants (Breviglieri et al., 2013; Howe, 1979; Thies et al., 2006), so visiting plants sporadically at night could reduce the chances of being predated.
The study of resource partitioning in space and time is vital to understand how interspecific competition can allow the stable coexistence of species with similar ecological characteristics (Bonaccorso et al., 2007; Schoener, 1974). Nevertheless, the effect of time partitioning on intra-guild competition has been poorly studied. We found that temporal activity patterns of Neotropical frugivorous bats are variable between assemblages, sympatric species, and populations of the same species. Activity patterns respond to the interaction of multiple factors, ranging from the supply of resources to interactions with other species. The results suggest that the association between plant and bat genera (i.e., Carollia - Piper, Sturnira - solanum), which may reduce intergeneric bat competition, may partially explain the high overlap in the activity patterns we observed. Besides, although the highest activity is conserved at the beginning of the night, the phylogenetic factor does not seem to influence the conservation of the activity patterns in bats, unlike other groups such as rodents (Castro-Arellano & Lacher, 2009). Finally, we found that species with similar size, abundance, and evolutionary history differentiate their activity patterns. Thus, our study provides empirical support for a trend towards temporal segregation in the activity of ecologically similar species, which may represent a mechanism that promotes coexistence. Consequently, our results contrast with those pointing out that temporal activity patterns of sympatric species do not respond to interspecific competition.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


