Introduction
Management of commercially exploited species heavily relies on regulatory tools that protects the fishery resources by recovering overfished stocks, protecting fish habitat, regulating fishing gears, and minimizing bycatch (Magnuson-Stevens Fishery Conservation and Management Act, 2014). But to be effective, such regulations should be supported by reliable, up-to-date population-level estimations of the ecological and life-history parameters involved coupled to the ecosystem and human interactions (Pikitch et al. 2004; Townsend, 2019). In crustacean fisheries, the establishment of a legal minimum size and bans on females catching are the instruments traditionally applied, but often with no previous studies on the local populations to support it (Olson et al., 2018). Accurate estimation of the size range at which most individuals reach maturity is fundamental to set minimum legal sizes for extraction with the aim of leaving a portion of the population to reproduce at least once before being harvested. Particularly in crabs, the determination of maturity has been based on changes in molt increments, gonad development, detection of spermatophores in males and females or even size-specific behaviors, but by far the most used criterium is the identification of changes in the relative growth of different body parts known to be under sexual or fecundity selection, i.e., secondary sexual traits (Farias et al., 2020; Hartnoll, 1978; Lovett & Felder, 1989). The determination of maturity based on morphological changes termed “morphometric maturity” requires the proper analyses of the relative growth trajectories to detect significant artifact-free changes in body shape that mark the onset of maturity. There are several alternative methods to perform these analyses, each with its pros and cons (Farias et al., 2014; Farias et al., 2020; Hartnoll, 2012). All methods are based on the same reasoning that if there is one conspicuous change in the shape of a body part known to be involved in reproduction, such change should reflect the onset of sexual maturity (or the so-called ‘size at morphometric maturity’ since it was estimated based in morphometric data). The typical body parts used for this aim are the male claws involved in the intra-sex competition for mating, courtship, and pair handling during copula, and the female abdomen, which widens through the ontogeny to protect and carry the eggs until spawning (Hartnoll, 2012).
Cardisoma guanhumi is a land crab species that inhabits the coastal forests, mangroves, wetlands, grasslands in the Caribbean islands, and tropical and subtropical regions of North, Central, and South America (Chase & Hobbs, 1969). This species has a key ecological role in the tropical coastal forest ecosystems, modifying soil properties with its digging activities (Quintero-Torre et al., 2018; Ridd, 1996), and affecting the seedling density and recruitment of the mangroves (Lindquist et al., 2009; Sherman, 2002). Therefore, the conservation of herbivorous land crabs should be included in management plans to maintain the forest structure, composition, and function (Lindquist et al., 2009).
Despite a steep decline in number over the past decades, likely due to habitat change and overexploitation (Govender, 2007; Rodríguez-Fourquet & Sabat, 2009), C. guanhumi still sustains a great deal of artisanal and commercial activity so that its harvest is both economically and culturally relevant (García-Quijano et al. 2015a, García-Quijano, et al. 2015b). However, an earlier perceived population decline related to a reduction in the reported landings provoked the Puerto Rico Department of Natural Environmental Resources (DNER) to regulate the species’ capture by prohibiting the capture of gravid females by imposing an arbitrary size limit of 64 mm carapace width (CW) for both sexes, and by establishing a temporary ban on the capture during the reproductive season of the crab (Reglamento de Pesca de Puerto Rico, 2010). Within the broader aim of generating basic biological information needed for the development of more sustainable exploitation of C. guanhumi in Puerto Rico, we studied the size structures and sex ratio in different areas and model the relative growth of body structures considered to be under sexual and fecundity selection with the ultimate aim to estimate the size at first maturity of both males and females. We discuss our results in contrast to the available information for the species and comparing the studied areas, suggesting changes to the parameters used by current local regulations.
Materials and methods
Sampling and study area: Sampling was conducted between 2001 and 2020 at nine different coastal locations around Puerto Rico, in the municipalities of Fajardo, Ceiba, Guánica, Cabo Rojo, Manatí, and Maunabo (Fig. 1). Each sampling event consisted of collecting crabs using traps set within fixed 10 x 10 m plots established a priori with that aim, from now on referred to as “sampling sites” or only “sites.” The traps were made of PVC pipes of different diameters (4, 5, 8, and 10 cm) that allow the crabs to enter the tube and then block the exit with a lid (Fig. 2A). The traps were placed in all burrows present within the sampling site for approximately 12 hours from sunset to sunrise, corresponding to the hours of most activity of the land crab (Feliciano, 1962; Taissoun, 1974), except in Manatí where they remain for six hours. For all specimens, the sex was determined, and the following measurements were taken using a dial caliper to the nearest ± 0.1 mm: the males’ and females’ carapace width (CW), major chela male’s propodus length (PL), and female’s fifth abdominal segment width (AW) (Fig. 2B).
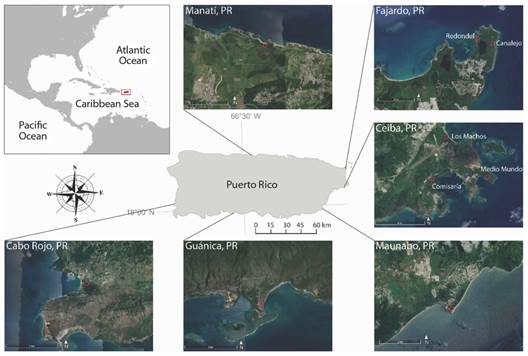
Fig. 1 Map of Puerto Rico, showing municipalities and sampling sites in this study. Satellite images retrieved from Google Earth (n.d.).
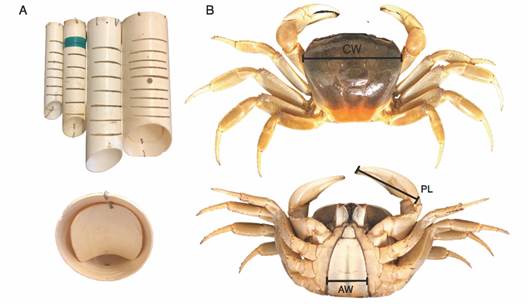
Fig. 2 A. Trap method used and B. Juvenile female of Cardisoma guanhumi showing where the morphometric measurements were taken. CW: Carapace Width, PL: Propodus Length in the major chela, AW: Abdomen Width.
There was only one sampling site per municipality, except in the two easternmost, Fajardo and Ceiba (latitudinally distant ca. 15 km from each other), in which two and three sampling sites were chosen respectively. The details of the sampling sites and periodicity are described below and summarized in Table 1.
TABLE 1 Numbers of individuals of Cardisoma guanhumi, sampling sites and collection and dates of collection
| Sampling site | Dates | Males | Females | Total |
| Redondel | May 2001 - November 2002 and February 2020 | 412 | 373 | 785 |
| Canalejo | June 2001 - November 2002 | 41 | 66 | 107 |
| Comisaría | June 2001 - November 2002 | 71 | 69 | 140 |
| Los Machos | June 2001 - November 2002 | 58 | 61 | 119 |
| Guánica | December 2001 - November 2002 and July 2017 | 92 | 92 | 184 |
| Cabo Rojo | June 2001 - November 2002 and March 2020 | 188 | 203 | 391 |
| Manatí | April 2006 - February 2020 | 546 | 422 | 968 |
| Maunabo | December 2017 - April 2019 | 86 | 57 | 143 |
| Medio Mundo | July 2017 | 10 | 2 | 12 |
| Grand Total | 1 504 | 1 345 | 2 849 |
Sampling was continuous between the time ranges specified except for Manati.
Reserva Natural Las Cabezas de San Juan is located in the Northeastern part of Puerto Rico in Fajardo. Two sampling sites, Redondel and Canalejo, are within the 178 ha of the reserve, where the mean annual temperature and precipitation are 25.6 ºC and 1 709.2 mm respectively (https://www.ncdc.noaa.gov/cdo-web/). Redondel is located 90 m inshore and has been used as barren land/pasture converted into a mangrove/coastal forest and has been a coastal forest for over 20 years. In turn, Canalejo is 13 meters away from the ocean and has been a coastal forest for over 80 years (Rodríguez-Fourquet, 2004).
Former United States Naval Station Roosevelt Roads is located in the Eastern part of Puerto Rico in Ceiba. Three sampling sites, Comisaría, Playa Los Machos, and Medio Mundo y Daguao are within the 3 480 ha where the mean annual temperature is 27.1 ºC and mean annual precipitation is 1 329.44 mm respectively (https://www.ncdc.noaa.gov/cdo-web/). Comisaría is located 486 m inshore and is a swamp with marsh deposits with a land-use history that includes agriculture and pasture, secondary forest, military facility, and mangroves. Playa Los Machos is located 55 m from the ocean and has been a beach with dune deposits and mangrove forest for more than 85 years (Rodríguez-Fourquet, 2004). Medio Mundo y Daguao is a coastal forest located 195 m from shore.
Reserva Natural Punta Ballena is located in the Southwestern part of Puerto Rico in Guánica. This sampling site is located within the 65.9 ha reserve, where the mean annual temperature and precipitation are 24-28 ºC and 762 mm, respectively (Guánica Dry Forest, unpublished data, 2004). This site is located at 534 m inshore and is a dry forest with abandoned plantation and secondary forest development. The land-use history includes barren land and mudflat (Rodríguez-Fourquet, 2004).
Refugio de Vida Silvestre de Boquerón is located in the Southwestern part of Puerto Rico in Cabo Rojo. This sampling site is located within 182 ha of the refuge, where the mean annual temperature and precipitation are 25.9 ºC and 594 mm, respectively (Boquerón Wildlife Refuge). This site is located 594 m from the Rincon Lagoon and is composed of dense forest mangrove. The land-use history of the area changed from agriculture to dense forest mangrove surrounded by an abandoned landfill and pastures (Rodríguez-Fourquet, 2004).
Reserva Natural Hacienda La Esperanza is located in the Northern part of Puerto Rico in Manatí. The reserve has an area of 898.5 ha where the mean annual temperature and precipitation are 25.0 ºC and 1 443.48 mm, respectively. The land-use history of the area has been sugar cane, pasture, and most recently, an increase in wetlands and coastal forest has been observed (Fideicomiso de Conservación de Puerto Rico, 2011).
Reserva Natural Humedal Punta Tuna is located in the Southeastern part of Puerto Rico in Maunabo. This sampling site is located within 43.2 ha of the refuge, where the mean annual temperature and precipitation are 26.6 ºC and 1 833.4 mm, respectively. This site is located at 110 m inshore, and the land-use history of the reserve included swamps, agriculture, drainage of swampy areas, pasture, and coastal forest (Estudios Técnicos Inc., 2009).
Data analysis: The size structure was first visualized by building histograms separated by sex and location using carapace width as the variable for absolute size. Then sexual differences in size distributions were evaluated using Kernel Density Estimators (KDE) following Farias et al. (2014). This method allows knowing whether the differences are due to distributions’ shapes, position, or both. A chi-square was also performed to identify differences in the sex ratio of each location, below and over the minimum legal size (MLS).
First, the whole morphometric dataset was pooled, and outliers were eliminated after confirming that they represented limb regenerations. Then the relative growth of selected body parts was modeled, and morphometric maturity was determined using the method developed by Watters and Hobday (1998) and used as in Farias et al. (2014). The method involves the use of smoothed splines instead of parametric models such as the classic two segment model of Somerton (1980) or the other available variations of it (Corgos & Freire, 2006). The rationale behind using splines is to avoid fitting models on transformed data or based on a priori assumptions on the ‘real’ trajectory of the relative growth that may result in artificial breakpoints in the growth trajectories, misleading conclusions and estimations of the size at maturity (Farias et al., 2014, Farias et al., 2020; Packard, 2012). Briefly explained, the method consists in first ‘binning’ the bivariate data to create a new dataset with the values of the mean of each size class produced as the predictor variable and the median size of the body part of interest as the response variable. Then, a smoothed spline is fitted with degrees of freedom (d.f.) chosen based on a General Cross-Validation criterion. If the selected spline model is different from a straight line (have more than two d.f.), it is used to estimate the size at morphological sexual maturity by calculating the maximum point in its first derivative. This is made on the understanding that the first derivative of the relative growth trajectory represents the instantaneous growth rate for the average individual, so the maximum corresponds to the maximum rate of change in the relative growth of the selected body part. All the statistical analyses and the morphometric sexual maturity estimation were performed using the R software version 3.6.1 (R Core Team, 2016).
Results
Size frequency distributions by sites and sex: A total of 2 849 crabs were captured during the study period. Twelve crabs from Medio Mundo y Daguao were not included in the size-frequency distribution analysis because this site was sampled only once. Of the 2 849 crabs, 1 494 males and 1 343 females (2 837 crabs) were used for the size-frequency distribution analyses. Most of the crabs (62 %) were captured in Redondel and Manatí (28 and 34 % respectively). Most crabs in Redondel were below the MLS in contrast with Manatí (Fig. 3). Only in Manatí were all sizes well represented in the two sexes, ranging from 15.9 to 118.5 mm CW in males and 15.4 to 106.6 mm in females. The remaining sites, with comparatively much fewer individuals sampled, had distributions skewed to sizes either smaller (Guánica, Cabo Rojo, and Maunabo) or larger (Canalejo, Los Machos, and Comisaría) than the MLS (red line in Fig. 3), and varied size range widths. Particularly, in Los machos and Canalejo, individuals smaller than 50 mm CW were absent. In general, some sites presented smaller crab sizes than in others (e.g. Guánica, Redondel and Cabo Rojo), and in others juvenile crabs were not found (e.g. Canalejo and Los Machos).
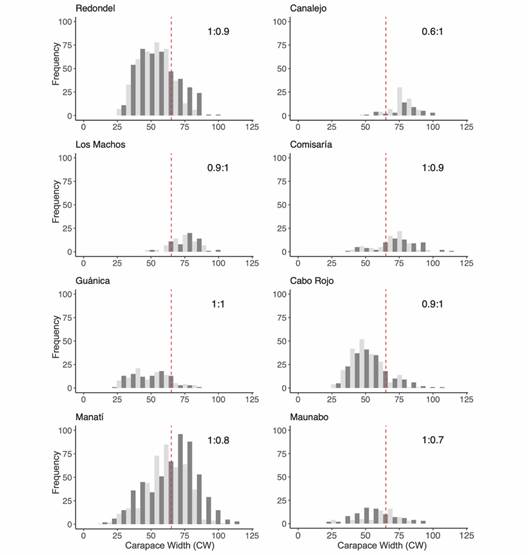
Fig. 3 Carapace width distributions for sampling sites in Puerto Rico. The sex ratio is shown in the upper right corner (Males:Females), and the red line shows the minimum legal size (MLS) in Puerto Rico (Dark Gray = Males, Light Gray = Females).
Size frequency distributions were mostly unimodal but, in some cases, differed between sexes and/or among locations (Fig. 3). When the distributions were tested with the KDE test, the populations of Redondel, Canalejo, Maunabo and Manatí show a difference in the position of their distribution by sex (KDE test, P < 0.05, Fig. 3). Redondel, Canalejo and Manatí show larger males than females. Maunabo is an atypical case because it presents more small males than females and the largest number of individuals over 60 mm are female. The only population that presents differences in both position and distribution is Manatí. This population shows practically a normal distribution for females. However, the distribution of male crabs shows a slight skewness to smaller sizes, showing that most of the male crabs have carapace widths greater than 60 mm. On the other hand, the populations of Cabo Rojo, Comisaría, Los Machos and Guánica do not present differences in position or the form of their distribution by sex (KDE test, P > 0.05, Fig. 3).
The sex ratio did not differ significantly between all populations (X2 = 4.37, d.f. = 7, P = 0.74). However, we found a significant difference in the sex ratio (1:1) within some populations. We found a difference over the MLS in the Redondel, Canalejo and Manatí populations (Table 2). The population of Redondel and Manatí has a greater number of males than females, while Canalejo shows a greater number of females. Also, a difference in sexual ratio was found below the MLS in Maunabo. This population shows a greater number of males below 64 mm CW. No differences were found in sex ratio in Los Machos, Comisaría, Guánica and Cabo Rojo (Table 2).
TABLE 2 Results obtained in the Chi-Square and Kernel Density Estimation (KDE)
| Site | Chi-Square Test | KDE Test (P) | ||||
| ≤ 64 mm CW | > 64 mm CW | Position and Shape | Only Shape | |||
| X2 | P | X2 | P | |||
| Redondel | 0.015 | 0.901 | 9.092 | 0.003* | < 0.001* | 0.016* |
| Canalejo | 1.600 | 0.206 | 8.670 | 0.003* | 0.040* | 0.854 |
| Comisaria | 0.053 | 0.819 | 0.160 | 0.689 | 0.052 | 0.482 |
| Los Machos | 0.571 | 0.450 | 0.036 | 0.850 | 0.774 | 0.830 |
| Guánica | 0.312 | 0.576 | 1.815 | 0.178 | 0.490 | 0.740 |
| Cabo Rojo | 0.884 | 0.347 | 0.063 | 0.803 | 0.560 | 0.880 |
| Manatí | 0.649 | 0.420 | 21.891 | < 0.001* | < 0.001* | 0.002* |
| Maunabo | 13.764 | 0.0002* | 0.891 | 0.345 | < 0.001* | 0.178 |
The chi-square evaluates differences in sexual ratio below and over the minimum legal size (MLS), and the KDE compares differences in the distribution by sex. *: Show significant differences.
Relative growth and morphometric sexual maturity: Propodus and abdomen data were obtained from a total of 318 crabs (162 males and 156 females), which were analyzed to estimate the morphometric sexual maturity size of this species in Puerto Rico. In males, the carapace width and propodus measurements were used to estimate morphometric sexual maturity obtaining the best spline model fitted to the data with four d.f. (Fig. 4). Using this model, males show positive allometric growth (b = 1.31). The first derivative shows the increase in the growth velocity of the propodus in a constant way up to 80 mm carapace width approximately. The major change in relative growth for males occurred at 77.6 mm (95 % CI 57.9-79.0 mm); this measurement corresponds to the mean size of morphometric sexual maturity.
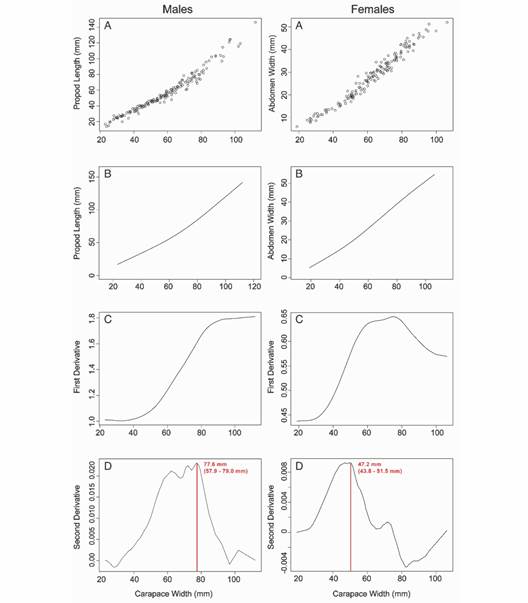
Fig. 4 Relative growth model and estimated morphometric sexual maturity for Cardisoma guanhumi in Puerto Rico. A. Original Data. B. Spline Model. C. First Derivative Plot. D. Second Derivative Plot with the sexual maturity size and 95 % confidence interval.
In females, the carapace width and abdomen measurements were used to estimate the morphometric sexual maturity obtaining the best spline model with four d.f. (Fig. 4). The female abdomen also showed positive allometry (b = 1.36). The first derivative shows that the abdomen has an increase in growth velocity until it reaches approximately 60 mm carapace width, where it then begins to decrease. For females, the morphometric sexual maturity occurs at 47.2 mm (95 % CI 43.8-51.5 mm). Analysis of female abdomen growth shows a decrease in growth velocity starting at 30 mm carapace width. Female propodus growth is isometric (b = 1.07) with a best spline model with two d.f. This model was not used for the determination of morphometric sexual maturity.
Discussion
Size distribution and sex ratio: Unimodal size distributions such as those found here are common in decapod populations (Díaz & Conde, 1989; García & Mantelatto, 2001; Sastre, 1991; Spivak et al., 1991). However, marked differences in both the position and shape of the unimodal size structure are not frequent among populations sampled from different sites with similar environmental features, as is the case here. Skewed distributions lacking individuals of a particular size range reflect particular processes affecting the local demography, such as punctual recruitment and mortality events, sustained size-selective harvesting, or habitat segregation (Gulland & Rosenberg, 1992). Populations of Comisaría, Los Machos, and Canalejo had mostly large individuals and no individuals below 40 mm CW, indicating past very low or episodic recruitment and relatively high survival rates of large individuals (Rodríguez-Fourquet, 2004). In contrast, Redondel, Guánica, Cabo Rojo, and Maunabo had size-frequency distributions skewed to the right, showing a relative lack of adult crabs above the MLS, probably due to land-use change (Estudios Técnicos Inc., 2009; Rodríguez-Fourquet, 2004) aggravated by illegal harvesting (C. Rodríguez-Fourquet, personal observation, 2018).
Redondel and Canalejo, which are in the same reserve and less than 1 km apart, have contrasting size structures, which interestingly look complementary. It seems that the larger crab sizes, particularly the males, that are scarce in Redondel are well represented in the very close and well-connected site of Canalejo, suggesting habitat segregation by size for both sexes. Land use in Canalejo has not changed over the past 80 years, while Redondel suffered many land-use changes during that period as previously described. High habitat disturbance associated with land use might be the reason for the lack of larger crabs in Redondel. It has already been shown that larger crabs are more abundant in areas with lower disturbances and less historical land-use changes (Rodríguez-Fourquet, 2004; Rodríguez-Fourquet & Sabat, 2009). Lastly, Manatí was the only sampling site where the size distribution structure resembled the normal distribution theoretically expected for populations of long-lived species with similar recruitment and mortality rates over time (Gulland & Rosenberg, 1992). In this area, land use has been mainly agricultural with minimum disturbance to coastal areas and no neighboring urban development (Fideicomiso de Conservación de Puerto Rico, 2011), conditions that seem to favor the stability of C. guanhumi populations at that site.
When comparing by sex, both the ratios and size distributions vary among sites in a manner that also seems to be resulting from differences in harvesting. Maunabo with a much smaller geographic extension than the other sites had experience many land-use changes throughout the years (Estudios Técnicos Inc., 2009). Based on field observations, we assume that the impact of harvesting is more significant than in other sites. In fact, during the sampling period, we found evidence of illegal harvesting, some of them reported following the DNER wildlife officers’ intervention. The departure from the 1:1 sex ratio below MLS and the difference in sizes could indicate a sex-selection harvesting effect where male crabs are more likely to be selected for their larger size as has been explained previously (Quiñones-Llópiz & Rodríguez-Fourquet, 2019). At Maunabo, male crabs are more abundant in the smaller size classes while larger sizes are dominated by females. In some crab fisheries, it has been shown that if harvesting is heavily biased towards males (either because they are larger and thus more profitable, or because of arbitrary regulations based on the idea of maximizing egg availability), the sperm availability and quality can become a limiting factor for the effective reproduction and sustainability of the stock (Pardo et al., 2017; Sainte-Marie, 2007). The male-biased fishery can have significant implications for the reproductive biology of the species and consequently in the population survival. Manatí shows the same male-biased sex ratios; however, most crabs are sexually mature males capable of reproduction. Canalejo shows a female-biased sex ratio, possibly because this study area is very close to the sea, allowing easy access to the water for larval release during the reproductive season.
Given the wide variation in the size structures we observed, even between very similar and close sites, it is evident that monitoring the populations over time can be informative. The monitoring will provide information about the natural demographic processes of the species that are not yet properly known, the degree of exploitation, and habitat recovery. This becomes very useful data in the current context of unreported artisanal and personal consumption extraction that limits the access to the catch effort data required for management decisions.
Sexual maturity using relative growth: The relative growth of the land crab Cardisoma guanhumi has been studied previously on populations in Florida, USA, showing sexual dimorphism (Gifford, 1962; Herreid, 1967). As previously described, the species present heterochely in both males and females, but males develop larger chelae in relation to body size. In crabs, heterochely and sexual differences in claw size are thought to be associated with the multiplicity of functions played by the chelae and the differences in use by sex, such as in defense, feeding, intra-sex antagonisms, courtship display, burrowing, mate guarding, and pair manipulation during copula (Daleo et al., 2009; Yamaguchi et al., 2005). In some land crab species, such as most fiddler crabs, the drivers for the development of sexual differences in relative growth of the chelae are well understood because of their role on male’s courtship, defense of the territory, and combat (Crane, 2015). However, in C. guanhumi, the specific function of the major chela was not studied in depth, although it was suggested that it has implications in social behavior (Herreid, 1967). The male size at sexual maturity found in this study is close to that reported in the literature for other regions in the continent (Herreid, 1967; Shinozaki-Mendes et al., 2013). The higher acceleration rate of propodus growth indicates the moment the crab reaches maturity. During growth, male crabs invest much energy in gonadal development, and once their gonads mature, they can invest energy in the growth of other organs. This difference occurs after the prepubertal molt, previously described by Teissier (1960).
Females also present heterochely as discussed above. However, the isometric growth of the propodus with respect to carapace width makes it impossible to estimate the size of sexual maturity. The model used in this study permits estimating tipping points in the ontogenic trajectory of the relative growth only if it is significantly different from a straight line. Females often show allometrically positive growth of the abdomen during gonadal maturation and then a decrease in growth acceleration when they reach puberty (Hartnoll, 1988). The morphometric size at sexual maturity based on the abdomen found in this study is smaller than most previously reported in the literature (Botelho et al., 2001; Shinozaki-Mendes et al., 2013; Silva et al., 2014). However, the methods used differ among studies. Also, the size of sexual maturity may vary by location because growth in crabs is limited by several factors, for example, availability of water and food (Hirose et al., 2013; Spivak et al., 2016; Wolcott, 1988).
The exploitation of natural populations with a strong bias towards the removal of the largest individuals may lead to changes in the mean values of some life history and other features of the harvested population, e.g. the size at first maturity, maximum size and sex ratios. In particular, a reduction in the size at maturity due to size biased harvesting has been observed in variety of species form different taxa, including true crabs (e.g. Chionoecetes bairdi) (Edeline et al., 2007; Grift et al., 2003; O’Dea et al., 2014; Zheng, 2008). Therefore, the difference observed in the size at sexual maturity of the females compared to other regions can be an indication of the intense harvesting pressure that the species is experiencing in Puerto Rico. Early maturity increases the probability that the population will reproduce, at least once before being captured, in response to exploitation on the island. Regulation in Puerto Rico allows the capture of females that are non-ovigerous and exceeding a minimum size of 64 mm carapace width (Reglamento de Pesca de Puerto Rico, 2010). In practice, however, it is known that egg-bearing females are routinely caught due to the lack of proper control in the field and their higher value, as the egg masses are considered a culinary delicacy, increasing the possibility of changes in the life history of this species.
Implications and Recommendations: Current regulations in Puerto Rico afford protection to the female crabs but not to larger male crabs. The regulation establishes that crabs smaller than 64 mm carapace width cannot be captured. Therefore, the whole range of female sizes reaching morphometric sexual maturity is protected (range 43.8 to 51.4 mm CW). However, males that reach sexual maturity at a larger size are not protected by this regulation. The higher limit of the confidence interval of the morphometric sexual maturity is 79.0 mm carapace width (range 57.9 to 79.0 mm carapace width), indicating that sexually immature male crabs are legally captured under current regulations. This could have significant implications for the reproduction of the species and, therefore, for the conservation of its populations on the island. We suggest considering different size limits depending on the sex of the crab. Based on our estimates of morphometric sexual maturity for C. guanhumi in Puerto Rico, we recommend that the minimum commercial size be 60 mm CW for females and 80 mm CW for males. This will allow the full range of sizes where sexual maturity is reached to be protected, increasing the likelihood of the population’s size to increase.
Knowing the ecological, cultural, and economic importance of this species and the minimal information that exists, research and mitigation efforts should be increased on the island. Immediate measures should be imposed to ensure the protection of populations of this species as well as the habitat. We recommend this effort to go with an educational component that informs the public and community about the importance of this species to the coastal ecosystems in Puerto Rico and the danger they face. More research about the ecology, reproduction and physiological sexual maturity combined with the regulations will safeguard the populations of C. guanhumi for future generations.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


