Introduction
Loranthaceae Juss. is the most diverse family of parasitic plants in the tropics, comprising approximately 73 genera and 960 species (Dueñas-Gómez & Franco-Roselli, 2001; Nickrent et al., 2010; Dueñas-Gómez, 2015). The monophyly of the family is strongly supported by molecular and morphological evidence, with the molecular data also providing robust support for the genus Nuytsia R.Br. as sister to the rest of the family (Vidal-Russell & Nickrent, 2008a; Nickrent et al., 2010; Su et al., 2015). In the aforementioned studies, Nuytsia forms a grade with two other early-diverging genera: (Nuytsia (Atkinsonia F.Muell. (Gaiadendron G. Don (rest of the Loranthaceae)))), although Nickrent et al., (2019) recovered an alternate topology: (Nuytsia (Gaiadendron (Atkinsonia (rest of the Loranthaceae)))). Grimsson et al., (2017) recovered a completely different topology in which Atkinsonia was resolved as sister to an entirely Australasian clade, but with a low bootstrap support value. More data are desirable to resolve the divergence order in this basal part of the tree.
The current classification of the family includes five tribes and 11 subtribes (Nickrent et al., 2010; Kuijt, 2015). This study focuses in tribe Nuytsieae Tiegh., including only Nuytsia floribunda (Labill) R.Br., endemic to Western Australia, and tribe Gaiadendreae Tiegh., comprising Atkinsonia ligustrina (Lindl.) F. Muell., also from Australia, plus the Neotropical Gaiadendron, which includes G. punctatum (Ruiz & Pav.) G. Don, distributed in high montane ecosystems of South and Central America, and G. coronatum Kuijt, narrowly endemic to high-Andean dwarf forests in Peru (Kuijt & Graham, 2015). Although Gaiadendreae are not monophyletic, its two component genera share the following characters: versatile, dorsifixed anthers, fruits forming pseudodrupes and seeds with sulcate endosperm. The inflorescences of Atkinsonia are composed of monads, while those of Gaiadendron form triads, but the floral morphology is similar in both genera (Nickrent et al., 2010).
The first embryological studies in the family, including the first interpretation of the reproductive organs, were published by Treub (1883). A century later, Bhatnagar and Johri (1983) studied the floral structure and embryology of an expanded sample of 13 genera, culminating in the publication of their series titled “Morphological and embryological studies in the family Loranthaceae”. Contemporaneously, Cocucci (1982) and Venturelli undertook studies of some Neotropical genera including Struthanthus Mart. (Venturelli, 1981) and Tripodanthus (Eichl.) Tiegh. (Venturelli, 1983). Based on their work, these authors had different interpretations of the process of reduction of the gynoecium and ovules observed in the family (Cocucci, 1982; Bhatnagar & Johri, 1983). The “primitive” condition was represented by Nuytsia (Bhatnagar & Johri, 1983) or by Tripodanthus and Nuytsia (Cocucci, 1982), in both cases with ategmic ovules completely separated from the ovary wall and enclosed in differentiated locules with the embryo sacs pointing towards the base of the gynoecium.
These interpretations were based on detailed studies of embryogenesis in Nuytsia floribunda (Narayana, 1958a). Detailed embryological studies have also been conducted on Atkinsonia ligustrina by Prakash (1961), who suggested that it would be useful to study the embryology of Gaiadendron in order to test the relationships of the Gaiadendreae and Elytrantheae Engl. (considered subtribes at that time). More recently, Suaza-Gaviria et al., (2016) studied the floral structure of Gaiadendron along with that of other Neotropical Loranthaceae species, but this work was mostly focused on inflorescence morphology.
Here we present a detailed study of floral development in Gaiadendron punctatum in order to fill in existing gaps in the data and thereby allow direct comparison of floral morphology and especially embryological characters among the three basal genera (Nuytsia, Atkinsonia and Gaiadendron) in Loranthaceae, data that may help shed light on the as-yet unresolved relationships among these taxa.
Materials and methods
Plant material: Flowers at different phenological stages (buds, anthetic and post-anthetic flowers) were collected in the field and immediately fixed in FAA (formaldehyde: acetic acid: 70 % ethanol, 10:5:85) from a plant of a small population of Gaiadendron punctatum from the following locality: Colombia. Dept. Cundinamarca, Municipality la Calera, locality “Los Patios”, North of the tollboth, paramo ca. 3 000 m. (voucher specimen Raz 1742, COL). The determination was confirmed using keys and descriptions by Kuijt (1981) and, Dueñas-Gómez and Franco-Roselli (2001).
In order to facilitate the description of the floral developmental processes, we established six stages of flower development taking into account the phenological phase (bud: 1 to 4; anthesis: 5; post-anthesis: 6), the total length of the flower measured in longitudinal section and the observed phases of microgametogenesis and megagametogenesis in the androecium and gynoecium, respectively (Table 1).
TABLE 1 Floral developmental stages studied in Gaiadendron punctatum, lengths in mm and characteristics associated with the androecium and gynoecium
| Stage | Length (mm) | Characteristics | |||||
| Inferior ovary | Calyculus to apex | Total | Style and stigma | Androecium | Gynoecium | ||
| 1 | 1.7 | 2.5 | 4.2 | 1.5 | Archesporium, dyads and tetrads | Ovules | |
| 2 | 2.1 | 3.4 | 5.5 | 2.0 | Tetrads | Meiosis in the ovule | |
| 3 | 3.1 | 5.4 | 8.5 | 4.2 | Tetrads, tetrads with triradiate pollen | Meiosis in the ovule | |
| 4 | 3.5 | 6.5 | 10 | 4.8 | Mature pollen free uninuclear and binuclear | Lengthening of the embryo sacs | |
| 5 | 3.8 | 8.1 | 11.9 | 6.4 | Anthesis flower, anther dehiscence, mature pollen shed | Lengthening of the embryo sacs | |
| 6 | 4.0 | - | - | 9.5 | Post-anthesis flower without petals and stamens | Mature embryo sacs, Complete ovular apparatus | |
The flowers, fixed in FAA for 48 hours, were subsequently stored in 70 % ethanol, and treated following the Robles et al., (2016) protocol. This involved standard methods of dehydratation, paraffin infiltration, sectioning with a rotary microtome (820 Spencer, American Optical Company, NY) and attachment to microscope slides. The slides were stained with astra-blue and fuchsine and deposited in the Department of Biology collection at the Universidad Nacional de Colombia, Bogotá. The slides were analyzed and photographed using an Olympus BX-50 microscope with a Moticam Pro 282B camera. Fixed flowers were studied with a Leica M205A stereoscope using multifocus mode and photographed with a Leica MC 170 HC camera. Digital images were processed and edited with the software Adobe Photoshop CC and InDesign.
Results
Flower and inflorescence morphology: Gaiadendron punctatum is a profusely branched shrub with axillary and/or terminal compound racemose inflorescences, developing acropetally (Fig. 1A). The partial inflorescences are dichasia composed of a central flower and two sessile lateral flowers (Fig. 1B). The dichasia are opposite and decussate along the inflorescence axis, each subtended by bracts. All the flowers are bisexual and subtended by foliaceous bracts.
The flower of Gaiadendron punctatum is epigynous, so the ovary is inferior (Fig. 1B, Fig. 1C) and is surrounded by the hypanthium. The inferior ovary subtends the other floral whorls: the calyx (or calyculus), the corolla and the androecium of epipetalous stamens (Fig. 1D).
The anatomical description presented here follows a topological order, from the base to the distal parts of the flower. We begin with the receptacle, then the inferior ovary and the hypanthium, following the vascularization as it continues into the style and stigma and the perianth: calyculus and corolla, ending with the epipetalous androecium. The processes of microsporogenesis and megasporogenesis are described in association with the description of the gynoecium and androecium, respectively.
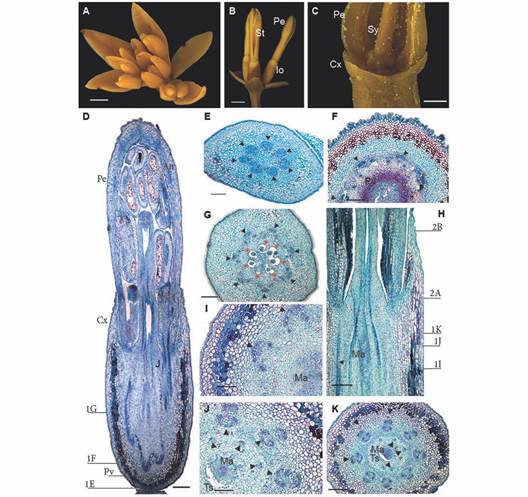
Fig. 1 A. Inflorescence and flower anatomy of Gaiadendron puntantum. A. Inflorescence with flowers developing acropetally; B. Lateral flowers in side view; C. Detail of flower with inferior ovary; D. Longitudinal section of the flower (the lines indicate the approximate position of the transverse sections corresponding to Fig. 1E, Fig. 1F and Fig. 1G); E. Transverse section (TS) of the floral receptacle; F. TS at the pelvis level; G. TS at the ovary level; H. Detail of the mamelon in longitudinal section (the lines indicate the approximate position of the transverse sections corresponding to Fig. 1I, Fig. 1J, Fig. 1K, Fig. 2A and Fig. 2B; I. J. and K. TS at different levels of the mamelon immersed in amyliferous tissue. An = Anther; Cx = Calyculus, Io = Inferior ovary; Ma = Mamelon; Pe = Petal; Pv = Pelvis; Sy = Style; St = Stamen; Ts = Surrounding tissues; Triangle = Vascular bundle; Triangle with number 1 = External vascular bundle; Triangle with number 2 = Internal vascular bundle; Red asterisks = Locules; D. E. F. G. H. I. J. and K. Astra blue/fuchsine. Scale bars: 2 mm in A. and B.; 1 mm in C.; 500 µm in D.; 300 µm in E.; 200 µm in F.; 150 µm in G.; 100 µm in H. I. and J.
Gynoecium and floral vasculature: Seven traces enter the sessile flowers via the receptacle from the inflorescence axis (Fig. 1E). The mature hypanthium has a single epidermal layer composed of elongate, papillose cells covered by a thick cuticle; after the epidermis, 6-9 inner layers of tanniniferous parenchymatic tissue are observed, followed by 10-11 layers of parenchyma towards the central region; the vascular bundles that irrigate the petals and stamens are embedded in parenchymatic cells (Fig. 1F).
The ovary is situated above the base of the receptacle, which is in turn composed of sclerenchymatic tissue (when mature) and is referred to as the pelvis (Fig. 1F). It is composed of seven basal locules each with a single ategmic ovule (Fig. 1G). There is a non-vascularizated conical structure above the locules called the mamelon (Fig. 1H) which is composed of amyliferous parenchyma that is fused with the surrounding tissues; embryo sacs will eventually grow through this tissue (Fig. 1I). The mamelon is separated from the surrounding tissues just beneath the style (Fig. 1J, Fig. 1K).
At the base of the flower, seven vascular bundles can be seen forming a ring at the periphery of the pelvis (Fig. 1E); above this point, the bundles divide to form two concentric rings (Fig. 1H, Fig. 1I). The interior ring is made up of seven bundles that correspond to the seven locules of the gynoecium (Fig. 1H, Fig. 1J); all or part of these five to seven bundles continue into the style (Fig. 2A, Fig. 2B). There are seven vascular bundles in the exterior ring, each one composed of three to five strands including a large central strand that is external to the others, plus two to four lateral strands (Fig. 1I, Fig. 1J, Fig. 1K). The lateral strands fuse to form a new bundle that enervates the stamens, while the external strand enervates the petals. No vascular traces enter the calyculus (Fig. 1D, Fig. 1E, Fig. 1F, Fig. 1G, Fig. 1H), in which there is neither evidence of tracheids, nor any other vascular tissue.
Megasporogenesis: The ovary is composed of seven locules (Fig. 1G), each of which contains a single ategmic, tenuinucellate ovule oriented towards the base of the flower (Fig. 2D). The placentation is axillary. In floral developmental stages 2 and 3 (Table 1), meiosis can be observed, resulting in four cells that are similar in size and contour (Fig. 2E, Fig. 2F). In each locule, the developing embryo sacs elongate through the amyliferous tissue of the mamelon until they reach the base of the style (Fig. 2C, Fig. 2J; stage 6 in Table 1) without invading the latter structure.
At the upper end of the mature embryo sac, a small protuberance is observed. It is called the caecum, which functions as a haustorium (Fig. 2G). It is followed by the synergids, the filiform apparatus, the egg cell and the central cell (Fig. 2H). The antipodal cells are found at the base of the embryo sac, near the hypostase and these tend to degenerate (Fig. 2I).
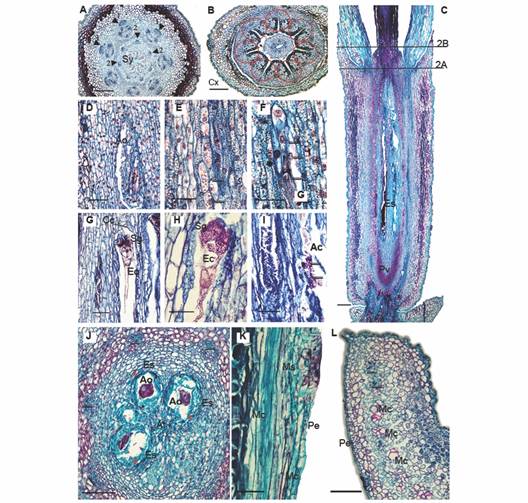
Fig. 2 Floral morphoanatomy of Gaiadendron punctantum, megasporogenesis and perianth. A. Transverse section of the flower (TSF) at the base of the style; B. TSF at the level of the calyculus; C. Longitudinal section (LS) of the gynoecium, (the lines indicate the approximate positions of the corresponding transverse sections in A. and B.); D. LS, detail of the ovary and ategmic ovule; E. LS after first meiotic division, arrows indicate nuclei; F. LS, after second meiotic division, arrows indicate nuclei; G. and H. LS at the upper level of embryo sac showing the caecum, synergids and egg cell; I. LS at the lower level of embryo sac showing degenerated antipodal cells; J. Transverse section at the level of the embryo sacs; K. and L. Detail of the mucilage secreting structures. Ac = Antipodal cells; Ao = Ategmic ovule; At = Amyliferous tissue; Cc = Caecum; Cx = Calyculus; Ec = Egg cell; Es = Embryo sac; Mc = Mucilage canal; Ms = Mucilage secreting structure; Pe = Petal; Pv = Pelvis; Sg = Synergids; Sy = Style. Triangle = Vascular bundle; Triangle with number 1 = External vascular bundle; Triangle with number 2 = Internal vascular bundle; Asterisks = Locules; Red circle = Interdigitated epidermal cells; 2e-3e = Second and third embryo sac divisions; A. B. C. D. E. F. G. H. I. J. K. and L. = Astra blue/fuchsine. Scale bars: 200 µm in A. B. and L.; 500 µm in C; 50 µm in D. G. I. and K.; 30 µm in E. F. and H.; 100 µm in J.
Style and stigma: The base of the style is expanded (Fig. 1C, Fig. 1D, Fig. 1H) and seven projections are observed at the periphery (Fig. 1C); these correspond to the outermost layers of the tissues that nourish the embryo sacs during their development (Fig. 2A). The style is hollow (Fig. 1D, Fig. 1H) and it is composed of a single outer epidermal layer interspersed with stomata and covered by a thick cuticle. Beneath the epidermis, there are 10-12 layers of tanniniferous parenchyma and seven collateral vascular bundles are observed towards the inner part of the tissue, surrounded by smaller parenchyma cells, which are, in turn, adjacent to the inner epidermis that surrounds the stylar canal (Fig. 2B).
There is a trilobate stigma at the end of the style, with interdigitated epidermal cells at the edges of the lobes; stigmatic cells are slightly rounded, whereas the epidermal style cells are flattened (Fig. 1D).
Perianth - calyculus and corolla: The calyculus is short and light green in color (Fig. 1C); it forms a ring above the ovary, being more or less persistent in fruit, with a variable number of irregular teeth at the margin (Fig. 1C, Fig. 2B, Fig. 2C). The epidermis is uniseriate abaxially and is made up of radially elongated cells and structures that secrete mucilage (Fig. 2B), the latter persisting even in fixed material (Fig. 1C). The adaxial epidermis (oriented towards the petals) is composed of quadrangular cells. The mesophyll is composed of undifferentiated parenchyma with small cells and thin walls as well as parenchyma with larger cells and dark, tanniniferous cytoplasm. As mentioned above, no vascular traces were observed (Fig. 2B).
The corolla is composed of seven long, white petals that measure approximately 3.5 x 1.3 mm, with valvate aestivation (Fig. 1B). The petals are postgenitally fused in the flower bud by interdigitation of the external epidermal cells (Fig. 1D, Fig. 2B, Fig. 3K). The bases of the petals are narrow and articulated (Fig. 1H). The corolla lobes are completely free at anthesis, but the basal third of the petals form a tube that surrounds the style; the petals are reflexed in the upper two thirds of their length, where the filaments of the epipetalous stamens are free from the petals (Fig. 3B). The adaxial epidermis of the petals is papillose, followed by 4-7 layers of tanniniferous parenchyma made up of very tightly packed cells; then there is a less compact parenchymatous region toward the center of the petals in which the vasculature is embedded, the latter consisting of a central bundle and one or two lateral bundles. Between the central and lateral bundles, some packets of sclerenchymatic cells with not very thick walls are observed; these are accompanied by cells that stain densely and form longitudinal mucilaginous ducts. Exterior to these cells, another layer of tightly packed parenchymatic cells is seen, some of which are tanniferous; the abaxial epidermis at outer edge of the petals is composed of quadrangular cells and mucilage-secreting structures (Fig. 2K, Fig. 2L).
Androecium and microsporogenesis: The androecium is composed of seven stamens of three different lengths; this variation can be seen in the mature flower as well as in earlier developmental stages (Fig. 3A). The filaments are adnate to the bases of the petals (Fig. 3B) and the anthers are dorsifixed (Fig. 3L, Fig. 3M), each with two thecae measuring 2-3 mm long (Fig. 3K). Two pollen sacs of similar size are observed within each theca, each with a uniseriate exothecium of very thin-walled, unlignified cells (Fig. 3C). The fibrous endothecium is made up of a single layer of large, quadrangular cells plus two thinner layers that do not persist, and a secretory tapetum that persists until maturity. The tapetum is non-invasive (Fig. 3I, Fig. 3K) and is initially composed of uninucleate cells, becoming binucleate at the end of their development. No amyloplast or other type of reserves were observed in the endothecium, or in any of the other tissues of the anther during its development.
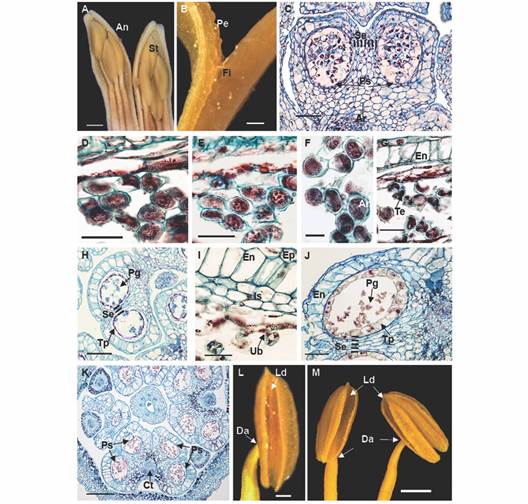
Fig. 3 Floral morphoanatomy of Gaiadendron puntantum: Androecium and microsporogenesis; A. Anther in longitudinal section in mature stage; B. Detail of the anther attachment; C. Immature pollen sacs with archaesporium (2n); D. Uninucleate microspores; E. First meiotic division; F. Dyads; G. Tetrad formation in the pollen sacs; H. Pollen sac with tetrads; I. Detail of fibrous endothecium, intermediate strata and tapetum; J. Pollen sac with mature pollen and persistent tapetum; K. Mature anther; L. and M. Dorsifixed anthers showing longitudinal dehiscence. An = Anther; Ar =Archesporium; Ct = Connective tissue; Da = Dorsifixed anther; En = Endothecium; Ep = Epidermis; Fi = filament of stamen; Is = Intermediate strata; Ld = Longitudinal dehiscence of the anther; Pe = Petal; g = Pollen grain; Ps = Pollen sac; Se = Septum; t = Stamens; Tp = Tapetum; Ub = Ubisch bodies (orbicules). C. D. E. F. G. H. I. J. and K. = Astra blue/fuchsine. Scale bars: 2 mm in A.; 500 µm in B., L.; 50 µm in C, J; 20 µm in D. E. G. and I.; 10 µm in F.; 100 µm in H.; 200 µm in K.; 1 mm in M.
Microsporogenesis begins with a solid, diploid archaesporium (Fig. 3C). The uninucleate microspores separate (Fig. 3D) and undergo two meiotic divisions (Fig. 3E), first forming dyads (Fig. 3F) and then tetrads (Fig. 3G). The four nuclei separate simultaneously, creating primarily tetrahedral tetrads (Fig. 3G), near which tapetum and orbicules (Ubisch bodies) can be observed, the latter appearing red in the figure. In the final stage, four trilobate pollen grains can be distinguished within the callose wall of the tetrad (Fig. 3H, Fig. 3I). The free pollen becomes binucleate just before anthesis (Fig. 3J).
Anther dehiscence is longitudinal and occurs via dehydration of the tissues (Fig. 3L, Fig. 3M). The cells of the exothecium near the stomium become elongated in the later developmental stages of the anther to facilitate pollen dispersal. Dehiscence occurs without prior coalescence of the pollen sacs in each theca, via rupturing of the central septum and the walls of the two pollen sacs along their entire length (Fig. 3J).
Discussion
Given the lack of robust support for a specific branching order among the three basal genera in the Loranthaceae (Gaiadendron, Nuytsia and Atkinsonia) according to the phylogenetic proposals available for the family, we consider the utility of comparative floral morphoanatomy to help resolve these relationships. With the data reported here for Gaiadendron, such a comparison is now possible and is presented below. Table 2 summarizes some of the characters and their states in the basal genera of Loranthaceae: Nuytsia, Atkinsonia and Gaiadendron. Comparisons with some members of the nearby tribe Elytrantheae are also considered here.
TABLE 2 Comparison of characters among the three basal genera of Loranthaceae
| Character | Nuytsia | Atkinsonia | Gaiadendron |
| Inflorescence | Racemose | Racemose | Racemose |
| Parcial inflorescence | Dichasium with central flower bisexual, laterals pistillate | Monad subtended by three bracts, bisexual flowers | Dichasium of bisexual flowers |
| Central mamelon | Present | Present (¿?) | Present |
| Number of locules | 3 | 4 | 7 |
| Fusion of the mamelon and adjacent tissues | No | ¿? | Yes, in the middle |
| Tracheids in the mamelon | Yes | ¿? | No |
| Number of vascular bundles at the base | 7 | 7 | 7 |
| Number of bundles in the internal ring of the ovary and (style) | 6 (6) | 4 (4) | 7 (5-7) |
| Number of bundles in the internal ring of the petals and (stamens) | 7 bundles each with: 1 external (2 laterals that fuse) | 7 bundles each with: 1 external (2 laterals that fuse) | 7 bundles each with: 1 external (2 to 4 laterals that fuse) |
| Vascularization of the calyculus | Yes, 5 initial traces that anastomose | Yes; no traces enter from the main stele, but tracheids differentiate in each lobe | No |
| Ovules | Ategmic, oriented towards the base, tenuinucellar | ¿? | Ategmic, oriented towards the base, tenuinucellar |
| Style | With amyliferous tissue | ¿? | Without amyliferous tissue |
| Intrusion of the embryo sacs | Ovary and style | Ovary | Ovary |
| Hipostase composition | Collenchyma | Collenchyma | Sclerenchyma |
| Base of the style | With angular thickenings | With slightly rounded thickenings | With angular thickenings |
| Anthers | Epipetalous, Basifixed | Epipetalous, dorsifixed, Versatile | Epipetalous, dorsifixed, Versatile |
| Sources | Narayana (1958a) Narayana (1958b) | Prakash (1961) Garg (1958) Cocucci (1982) | This study |
The inflorescences of the three basal genera in the Loranthaceae are indeterminate racemes; the parcial inflorescences are dichasia in Gaiadendron, but these are reduced to monads in Atkinsonia. The flowers are generally bisexual, except in Nuytsia, where the central flower is bisexual and the lateral flowers are pistillate or staminate (Narayana, 1958a); lateral flowers abort in some cases in this genus.
Gaiadendron punctatum has a septilocular ovary, which is the greatest number of locules in the Loranthaceae. Nuytsia (Narayana, 1958a) and Lepeostegeres (Dixit, 1958) each have three locules, while Atkinsonia, Amylotheca (Raj, 1970), Lysiana (Narayana, 1958b) and Peraxilla (Prakash, 1960) each have four. The ovary is unilocular in the remaining genera of the family (Kuijt, 2015).Nuytsia, Gaiadendron and the genera of the tribe Elytrantheae all have a mamelon, being amyliferous in G. punctatum and in the tribe Elytrantheae (Johri et al., 1992) and not being amyliferous in Nuytsia (Narayana, 1958a). The mamelon in G. puntatum is retained within the ovary without entering the style, a condition also reported in Peraxilla Tiegh. and Amylotheca Tiegh. (Prakash, 1960; Raj, 1970), while in Nuytsia, Lysiana Tiegh. and Lepeostegeres Blume (Dixit, 1958; Narayana, 1958a; Narayana, 1958b), the mamelon invades the hollow style. In Nuytsia the mamelon remains separate from the adjacent tissue, but in Lysiana and Lepeostegeres this structure is fused with the ovary wall and the tissues of the stigma.
The mamelon of Nuytsia does not have amyliferous tissue, but the style does have amyliferous parenchyma. Thus, the embryo sacs can invade the style for quite a good part of its length. The embryo sacs of Lysiana and Lepeostegeres also enter the style due to the invasion of the amyliferous tissue of the mamelon into the lumen of the stylar canal (Bhatnagar & Johri, 1983). The embryo sacs of Gaiadendron, just like those of Peraxilla y Macrosolen (Blume) Rchb., develop only up to the base of the style because the mamelon is short and there is no amyliferous tissue in the style. The mamelon is fused with the ovary wall in Gaiadendron, a condition also seen in Lysiana and Lepeostegeres (Dixit, 1958; Narayana, 1958b) but not in Nuytsia. The aforementioned characters suggest that Gaiadendron has a greater affinity with the tribe Elytrantheae than with Nuytsia.
Although Cocucci (1982) indicated that Atkinsonia ligustrina has an amyliferous ovary like that found in genera of the tribe Elytrantheae, there is no direct evidence of this condition in Atkinsonia according to the studies cited by Cocucci (Garg, 1958; Prakash, 1961).
Calyculus, base of style and nectary: The vascularization of the calyculus is different in each of the three basal genera of Loranthaceae (Table 2). The absence of bundles or tracheids in the calyculus of Gaiadendron punctatum is the state found in the rest of the Loranthaceae, while only Nuytsia and Atkinsonia present alternative states. This result supports the Su et al., (2015) topology of (Nuytsia (Atkinsonia (Gaiadendron (rest of the Loranthaceae)))). It should be noted that the inconspicuous calyculus of G. punctatum contrasts with that of G. coronatum, in which the lobes are dentate, well differentiated, and persistent in fruit (Kuijt, 2015).
Diverse types of nectaries have been reported in Loranthaceae. In the Neotropical clade Psittacanthinae, (sensuNickrent et al., 2010), the nectaries often form a disk or ring between the base of the style and the base of the petals. This ring usually has stomata and subepidermal parenchyma with dark-staining cellular contents, as can be seen in Peristethium leptostachyum Kunth (Tiegh.) (Robles et al., 2016), Psitacanthus Mart. (Robayo et al., 2020), Passovia Karst., Aetanthus (Eichl.) Engl. and Oryctanthus Eichl. (Suaza-Gaviria et al., 2016). A different type of nectary found at the base of the petals has been described in Cladocolea Tiegh. (Cid-Villamil, 2006).
Nevertheless, it has been reported that the basal genera Nuytsia (Narayana, 1958a), Atkinsonia (Prakash, 1961) and few species of the Tribe Elytrantheae have thickenings at the base of the style (Amylotheca, Lepeostegeres: respectively, Dixit, 1958; Raj, 1970). In some of these cases, they have been interpreted as possible nectaries (Lepeostegeres, Nuytsia: respectively Narayana, 1958a; Raj, 1970) and only in Lepeostegeres, the presence of a dark-staining subepidermal tissue has been confirmed (Schaeppi & Steindl, 1942). Also, Lamilla et al., (2020) recently found that the base of the style conforms a nectary in Tristerix longebracteatus (Desr.) Barlow & Wiens, a basal member of the tribe Psittacantheae Horan Suaza-Gaviria et al., (2016) suggested that G. punctatum presents a nectary disk. However, no disk was observed in the present study (Fig. 1D, Fig. 1E, Fig. 1F, Fig. 1G, Fig. 1H; Fig. 2B, Fig. 2C). Our longitudinal sections of G. punctatum found a widened base of the style with some stomata in the epidermis and typical parenchyma beneath the epidermis (Fig. 1D, Fig. 2B); nor did we find sunken stomata at the base of style, and therefore the evidence is insufficient to confirm or reject that this structure is a nectary. In contrast, our research team found a widened style in Tristerix formed by an epidermis with abundant sunken stomata, underlain by parenchymatic tissue with dense cytoplasm and abundant intercellular spaces surrounding the vascular bundles (Lamilla et al., 2020). Nevertheless, the dark-staining subepidermal parenchyma at the base of the petals and stamens suggests the possibility of a different type of nectary (Fig. 2B).
Anther dehiscence: The endothecium in Loranthaceae can be fibrous or non-fibrous. When is it fibrous, it is composed of thickened cells that surround the pollen sacs, interrupted by cells that lack thickenings. The position of these weaker cells determines the type of dehiscence (Table 3).
TABLE 3 Anther dehiscence in Nuytsia, Atkinsonia, Gaiadendron and some genera of the tribe Elytrantheae
| Genus | Endothecium | Dehiscence type | Scheme of dehiscence | Source |
| Atkinsonia | Fibrous | A |

|
Prakash, 1961 |
| Nuytsia | Fibrous | B |
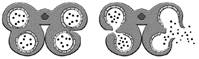
|
Narayana, 1958a |
| Gaiadendron | This study | |||
| Lysiana | Fibrous | C |

|
Narayana, 1958b |
| Peraxilla | Prakash, 1960 | |||
| Lepeostegeres | Non-Fibrous | D |

|
Dixit, 1958 |
| Amylotheca | Raj, 1970 |
In type A dehiscence, present in Atkinsonia ligustrina (Prakash, 1961), the two pollen sacs of each theca undergo coalescence due to the rupturing of the septum between them, followed by a single longitudinal rupture of the stomium.
In type B dehiscence, present in Nuytsia (Narayana, 1958a) and Gaiadendron (this study), the septum that divides the pollen sacs within each theca remains intact initially, and dehiscence occurs via simultaneous rupture of the septum and the outer wall of the theca (stomium), at the precise point where they connect, thereby creating a single longitudinal opening (Table 3).
In the genera Lysiana and Peraxilla of the tribe Elytrantheae, the interruption of the fibrous endothecium occurs in the middle of each pollen sac (Table 3) such that each theca opens via two longitudinal slits (type C dehiscence). On the other hand, in Amylotheca and Lepeostegeres, also of the tribe Elytrantheae, the endothecium has no thickened zones and therefore dehiscence may occur at any point (type D dehiscence).
According to Fig. 7F of Suaza-Gaviria et al., (2016), anther dehiscence in Gaiadendron punctatum occurs via two longitudinal slits in each theca, one for each pollen sac, corresponding to our type C. The results of the present study do not corroborate type C dehiscence in this taxon. The septum that separates the two pollen sacs within each theca is thickened and the weakest zone (Fig. 3K) is localized to the point where it fuses with the outer wall of the theca (the stomium, Table 3). Dehiscence occurs via a single longitudinal slit between the two pollen sacs (Fig. 3M). Therefore, Gaiadendron punctatum has type B anther dehiscence, just like Nuytsia floribunda.
Atkinsonia and Gaiadendron: clade or grade: In traditional classifications of Loranthaceae as well as in recent molecular systematic studies, Nuytsia, Atkinsonia and Gaiadendron have been considered basal genera. The character traditionally used to support this position is root parasitism through secondary haustoria, whereas in the rest of the family, it is the primary haustorium that parasitizes the stems of the host plants (Vidal-Russell & Nickrent, 2008b). It should be noted, however, that Gaiadendron punctatum can occasionally parasitize epiphytes (Kuijt, 2015).
The grouping of the genera Atkinsonia and Gaiadendron in the tribe Gaiadendreae was based on the following characters: vascular bundles in the calyculus, drupaceus fruit, furrowed endosperm and dorsifixed and versatile anthers (Nickrent et al., 2010). Regarding the first character, the vascularization of the calyculus occurs in three different states in each of these three basal genera (Table 2) with Gaiadendron punctatum lacking vascular traces or tracheids, a state shared with the rest of the Loranthaceae. It would be interesting to study this character in Gaiadendrum coronatum, the calyculus of which is much larger and persists in the fruit (Kuijt, 2015). Regarding the drupaceous fruit, Atkinsonia and Gaiadendron have pseudodrupes in which the embyro is surrounded by a hard endocarp. This condition is also found in fruits of the tribe Elytrantheae, including the genera Amylotheca (Raj, 1970), Lepeostegeres (Dixit, 1958), Lysiana (Narayana, 1958b) and Peraxilla (Prakash, 1960). The fruit of Nuytsia is completely distinct, being a winged nut (Kuijt, 2015). Discarding the first two characters, only furrowed endosperm and dorsifixed versatile anthers remain as possible synapomorphies of tribe Gaiadendreae as it is currently circumscribed.
In most molecular phylogenetic studies published to date (Nickrent et al., 2010; Su et al., 2015; Nickrent et al., 2019), Gaiadendron and Atkinsonia form a grade rather than a clade, although Grimsson et al., (2017) recovered Atkinsonia as sister to a clade comprising mostly genera of the tribe Elytrantheae. As can be seen, the morphological evidence is not unequivocal either, with some characters supporting Gaiadendron as sister to Atkinsonia (dorsifixed versatile anthers) and/or to Nuytsia (type of anther dehiscence), while the combination of characters 1. non-vascularized calyculus and 2. amyliferous mamelon would appear to support a sister relationship of Gaiadendron to the tribe Elytrantheae or to rest of the Loranthaceae. In order to definitively resolve the matter, it will be necessary to collect the last bits of missing data for Atkinsonia ligustrina (Table 2) and ideally, perform a combined analysis of molecular and morphological data to determine if the two genera of Gaiadendreae really do form a clade, a grade or are more distantly related.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


