Introduction
Water availability is one of the main environmental factors determining the establishment and distribution of tree species at local and regional scales (Engelbrecht et al., 2007; Markesteijn et al., 2011; Méndez-Alonzo et al., 2012). Different studies have shown that the performance of species varies in response to water availability, and this is explained through their functional traits (Engelbrecht et al., 2007; Poorter & Markesteijn, 2008; Markesteijn et al., 2011; González-M et al., 2020). For instance, species adapted to drought have developed functional traits that allow them to maintain growth despite low soil water potentials. Among the most studied traits are stomatal closure and leaf deciduousness, which allows plants to avoid dehydration during the driest periods of the day or year (Markesteijn et al., 2011; Méndez-Alonzo et al., 2012). Water storage in tissues to maintain xylem water transport and low levels of dehydration (Borchert, 1994; Scholz et al., 2007; Pineda-García et al., 2013), investment in deep roots to access more humid soils (Paz et al., 2015), or the presence of dense tissues that do not dehydrate and promote high resistance to cavitation (Bonal & Guehl, 2001; Comita & Engelbrecht, 2014). Despite this knowledge, studies are still needed that incorporate traits associated with other plant structures and functions, such as root traits.
Functional traits can be coupled to define functional strategies, and tree species exposed to water deficit are distributed along a continuum of traits related to tissue investment and a hydraulic safety-efficiency trade-off (Méndez-Alonzo et al., 2012; Pineda-García et al., 2015; González-M et al., 2020). At one extreme, there are species with high investment in dense tissues, dense stems with narrow xylem vessels, high leaf dry matter content, and leaf longevity. These species with dense and hydraulically safe tissues, known as “conservative” species, have low growth rates but high survival (Poorter et al., 2008). The other extreme are the “acquisitive” species characterized by thin, short-lived leaves with high investment in nutrients and less dense stems with wide xylem vessels. These soft tissues and their high hydraulic efficiency result in high growth rates but low survival (Poorter et al., 2008). Root trait dimension has been less explored. One hypothesis postulate that roots follow the “acquisition-conservation” trade-off analogous to leaf economic spectrum (Weemstra et al., 2016). However, recent studies have found mixed evidence (Valverde-Barrantes et al., 2015; Withington et al., 2006; Weemstra et al., 2016), showing decoupling between above and below ground traits (Withington et al., 2006; Weemstra et al., 2016; Freschet et al., 2020). Recent work showed a trade-off in C investment between deep roots and water storage tissues associated with successional and old-growth forest species, respectively (Paz et al., 2015). These studies invite to discuss the correlations between functional traits and whether coordination between traits reported in adults develops from the first stages of plant development.
Understanding the response mechanisms to drought is particularly relevant in seedlings because regeneration allows for the inference of forest successional trajectories and ultimately is key to determining forest resilience (Osuri et al., 2017). Additionally, seedlings are the most sensitive to environmental changes as they have poorly developed roots that do not reach deep soil layers (Engelbrecht & Kursar, 2003) and must compete for water and nutrients with established adult individuals (Lewis & Tanner, 2000). Furthermore, seedlings face light limitations in the understory that reduces their photosynthetic and growth rates (Romo, 2005); and in turn, they have limited carbohydrate reserves to invest in organs such as leaves or roots (Poorter & Markesteijn, 2008). These characteristics, added to the fact that many tropical species can spend decades under understory (Hubbell et al., 1999), make this ontogenetic state the bottleneck for establishing individuals and forests of the future.
Tropical dry forest is an ideal model for understanding how tree species cope with drought. First, it presents a strong dry season where soil water deficit limits tree species’ growth and establishment (Murphy & Lugo, 1986; González-M et al., 2019). Second, strategies to cope with water deficit have been well-studied in adult individuals, but it is unclear whether these strategies are fully developed from the seedling ontogenetic stage. And third, high fragmentation rates in this ecosystem can amplify the climatic severity due to the edge effects with effects on its structure and diversity (González-M et al., 2019). Understanding the functional mechanisms that determine seedling establishment will strengthen our ability to predict and understand these forests’ future under climate change scenarios in which greater seasonality and reductions in rainfall are expected (Dai, 2013; Feng et al., 2013).
This study evaluated the variation of 12 functional traits above and belowground in 38 species of dry forest seedlings. The selected traits are related to the different strategies of plants to cope with drought, such as investment in dense tissues, efficiency in root foraging of water and nutrients, and the water storage capacity (Markesteijn & Poorter, 2009; Méndez-Alonzo et al., 2012; Pineda-García et al., 2013; Paz et al., 2015; Salgado-Negret et al., 2016). The main objective was to identify the functional strategies and the correlation among pairs of traits belonging to different plant dimensions (leaf, stem, and root). We expected species are distributed along with two extreme strategies, acquisitive and conservative. However, we expect low coordination between above and below-ground functional traits due to a single conservation-acquisition trade-off cannot capture the variability of functions and environmental pressures to which the root system is subjected (Valverde-Barrantes et al., 2015; Weemstra et al., 2016).
Materials and methods
Study area and species selection: This study was carried out in a tropical dry forest located in Caribbean region in Colombia (Brasilar, municipality of San Jacinto, department of Bolívar - 9°53’40” N, 75°10’57” W), located at an altitude between 352 and 602 m a.s.l.
We selected 38 seedling species belonging to 32 genera and 21 families (14 deciduous and 24 evergreen species; Digital appendix 1). The species selection was based on their high abundance in the study area, and together they represented more than 80 % of the total abundance of seedlings registered in 96 plots of 1 m2 previously established for the area (Salgado-Negret, data unpublished). For abundant species, between 4 and 15 individuals (57.8 %) and rare species, between 1 and 3 individuals (42.1 %), were sampled. All individuals were located in similar light conditions (canopy cover varied between 72.1 % and 98.4 %) and similar relative soil humidity (6.36 and 21.1 %). The individuals had a height between 40 and 70 cm. Harvest of each seedling was carried out by digging with a shovel 50 cm around the stem to avoid losing the fine roots. The sampled seedlings were stored in plastic bags, labeled, and transported to the field station for processing.
Functional traits: In total, 12 functional traits belonging to the leaf, stem, and root dimensions were measured, following the protocols of Poorter and Markesteijn (2008), Pérez-Harguindeguy et al. (2013), Paz et al. (2015) and Salgado-Negret et al. (2016). Leaf traits were estimated for five leaves per individual. Each leaf was weighed fresh and scanned (Canon Canoscan LiDE 220) to estimate the leaf area (LA, mm2). Leaf thickness (LT, µm) was measured for each fresh leaf with a digital micrometer (Mitutoyo reference 293-240-30) in three different sections of the leaf, and avoiding the main veins. The leaves were dried at 70 ºC for 48 hours to estimate the dry weight, and from which the specific leaf area (SLA, cm2/g) and leaf dry matter content (LDMC, mg/g) were calculated.
Stem density (SD, g/cm3) was estimated by dividing the dry mass (dried at 70 ºC for 48 hours) and the green volume calculated by the water displacement method (Salgado-Negret et al., 2016). The stem water content (SWC, mg/g) was estimated by dividing the weight of the fresh water-saturated stem sample by the oven-dry weight. With the hydrated roots, the fresh weight of the whole root was estimated, then they were dried at 70 ºC for 48 hours to estimate the dry weight. Using an architectural criterion, the roots were separated into primary and secondary roots. The root dry matter content (RDMC, mg/g) was calculated as the relationship between the dry weight and the fresh weight of the secondary roots. The root water content (RWC, g/g) was determined as the relationship between the fresh weight and the dry weight of the primary root. The secondary-to-primary-root-mass ratio (SPRMR, g/g), was calculated as the secondary root mass per unit primary root mass (Markesteijn & Poorter, 2009). The aboveground-to- belowground -length ratio (AUL, cm) was calculated as the ratio between seedling height (cm) and maximum root depth (MRD, cm), both traits were estimated with a tape measure. Finally, leaf, stem and root mass fractions (LMF, SMF, RMF, g/g) were calculated as dry mass of each organ per unit dry plant mass). At the end, the LMF and SMF (LSMF, g/g) were summed as an indicator of the aboveground biomass of the plant.
To explore the relationships between pairs of traits, Pearson correlations using the Bonferroni correction were performed. In order to obtain an integrated view of the functional traits, a principal component analysis (PCA) was performed using the 38 species and the 12 functional traits to identify the functional strategies of the species. All analysis were performed using R statistical language (R Core Team, 2015).
Results
Significant correlations were reported between traits of different seedlings’ dimensions (Fig. 1). Functional strategies were observed in the PCA (Fig. 1, Table 1), and the first two ordering axes explained 61.4 % of the species’ variation as a function of the functional traits. PCA reported two functional continua; the first was dominated by the “trade-off” between resource conservation and acquisition. Conservative species showed high leaf and root dry matter contents (LDMC and RDMC) and high stem density (SD), while acquisitive species were related with high water content in roots (RWC) and stems (SWC) (Fig. 1). The traits that defined each of these strategies were negatively correlated (i.e., decoupled; Table 2). The second continuum separated the species with high investment in belowground biomass (RMF) and deep roots (MRD) from the species with tall height above ground (AUL), shallow roots, and high investment in secondary roots (Fig. 1, Table 2). Leaf traits such as SLA and LT were not correlated with other functional traits (Table 2).
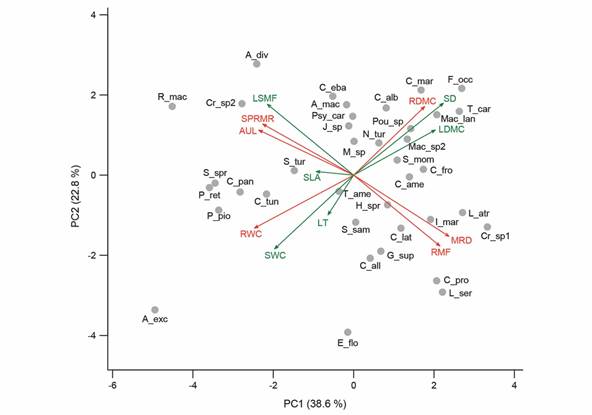
Fig. 1 Principal component analysis (PCA) of the average values of functional traits by species. Above-ground (green) and below-ground functional traits (red). The percentages of variation explained by the first two axes of the ACP are included in the graph. See trait abbreviations in Table 1.
Table 1 Loadings and proportion of variance of the principal component analysis of leaf, root and stem traits of 38 species of seedlings from a dry forest in Colombia
| Functional traits | Comp.1 | Comp.2 |
| LDMC | 0.29 | 0.225 |
| SLA | -0.133 | |
| LT | -0.198 | |
| SD | 0.319 | 0.359 |
| RMF | 0.307 | -0.353 |
| RWC | -0.353 | -0.264 |
| SPRMR | -0.323 | 0.255 |
| RDMC | 0.253 | 0.342 |
| AUL | -0.337 | 0.225 |
| SWC | -0.281 | -0.368 |
| MRD | 0.339 | -0.305 |
| LSMF | -0.307 | 0.353 |
| Proportion of variance | 38.6 | 22.8 |
Trait abbreviations: leaf dry matter content (LDMC), specific leaf area (SLA), leaf thickness (LT), stem density (SD), root mass fraction (RMF), root water content (RWC), econdary-to-primary-root-mass ratio (SPRMR), root dry matter content (RDMC), aboveground-to belowground length ratio (AUL), stem water content (SWC), maximum root depth (MRD) and leaf-stem mass fraction (LSMF).
Table 2 Pearson correlations with Bonferroni correction between leaf, root and stem traits of 38 species of seedlings from a dry forest in Colombia
| LDMC | SLA | LT | SD | RMF | RWC | SPRMR | RDMC | AUL | SWC | MRD | LSMF | |
| LDMC | 1 | -0.46 | -0.31 | 0.59** | 0.05 | -0.55* | -0.42 | 0.4 | -0.33 | -0.4 | 0.28 | -0.05 |
| SLA | 1 | -0.43 | -0.15 | -0.01 | 0.23 | 0.23 | -0.01 | 0.23 | 0.06 | -0.23 | 0.01 | |
| LT | 1 | -0.31 | 0.04 | 0.13 | 0.21 | -0.31 | 0.06 | 0.11 | 0.05 | -0.04 | ||
| SD | 1 | 0.07 | -0.74*** | -0.27 | 0.58** | -0.35 | -0.79*** | 0.19 | -0.07 | |||
| RMF | 1 | -0.30 | -0.60** | 0.2 | -0.49 | -0.11 | 0.68*** | -1*** | ||||
| RWC | 1 | 0.19 | -0.64*** | 0.32 | 0.74*** | -0.36 | 0.30 | |||||
| SPRMR | 1 | -0.05 | 0.71*** | 0.07 | -0.61*** | 0.60** | ||||||
| RDMC | 1 | -0.05 | -0.67*** | 0.05 | -0.2 | |||||||
| AUL | 1 | 0.2 | -0.79*** | 0.49 | ||||||||
| SWC | 1 | -0.14 | 0.11 | |||||||||
| MRD | 1 | -0.68*** |
*P <0.05, **P < 0.01, ***P < 0.0001. Trait abbreviations: specific leaf area (SLA), leaf dry matter content (LDMC), leaf thickness (LT), leaf-stem mass fraction (LSMF), stem density (SD), stem water content (SWC), root water content (RWC), root dry matter content (RDMC), root mass fraction (RMF), secondary-to-primary-root-mass ratio (SPRMR), aboveground-to belowground length ratio (AUL) and maximum root depth (MRD).
Discussion
Using 12 functional traits measured in 38 seedling species from a tropical dry forest, we found limited evidence of coupling between above and belowground traits, with the exception of tissue density (conservative strategy) and storage capacity of stems and roots (acquisitive strategy). Furthermore, roots’ traits relating to the ability to forage water and nutrients formed an orthogonal axis to the acquisitive-conservative continuum. These results show that root traits cannot be reduced to a single axis of variation, which is probably related to the multiple functions that they fulfill in the individual. Plants have different combinations of traits that enable them to deal with water limitations in these ecosystems.
The PCA did show that tissue density (SD, RDMC, and LDMC) was coordinated across plant dimensions, which has been reported previously in seedlings (Poorter & Markesteijn, 2008; Markesteijn & Poorter, 2009; Pineda-García et al., 2015; Weemstra et al., 2016). The coordination of high tissue density is an important strategy to cope with drought because species with dense stems are generally associated with narrow xylem vessels that allow them to reduce the risk of cavitation of the xylem (Markesteijn et al., 2011; Méndez-Alonzo et al., 2012). Additionally, a high density of fibers with thick cell walls may protect the xylem from the strong negative pressures generated by drought (Hacke et al., 2001). Dense, smaller, and more rigid leaves, have small transpiration surfaces that reduce the plants’ wilting and water requirements (Poorter et al., 2009). Examples of conservative species are Trichilia carinata (Meliaceae), Machaerium lanceolatum (Fabaceae), and Chiococca alba (Rubiaceae). Additionally, our results also showed coordination between water storage capacity in stems and roots. Water storage plants have low stem density which do not resist the xylem’s high tension during the dry seasons, which could have acted as a selection pressure promoting water storage in their tissues (Méndez-Alonzo et al., 2012). The root water storage is essential for the maintenance of photosynthetic and growth rates during dry seasons but also in the hours of greatest daytime transpiration when there may be a significant delay between the loss of water in the leaves and the absorption of water by the roots (Čermák et al., 2007). For the tropical dry forest, seedlings can use the water stored in the stems (SWC) to delay hydraulic failure for months (Pineda-García et al., 2013), and the high root water storage of seedlings (RWC) allows them to function physiologically in drought conditions (Poorter & Markesteijn, 2008). Piper piojoanum (Piperaceae), Piper reticulatum (Piperaceae), and Anacardium excelsum (Anacardiaceae) are examples of water storage species.
The orthogonal axis to the acquisitive-conservative continuum was related to the roots’ ability to forage water and nutrients in the soil. At one extreme, we found coordination in belowground traits related to explore and forage deep soils (high RMF and MRD). At the other end, species with shallow roots (low MRD), high secondary root biomass (high SPRMR), and high inversion on above ground length (high AUL) were located. Plants in dry forests have shown two main root strategies: deep roots with high carbon investment to explore the more humid soil horizons or investing in water-storing tissues (Paz et al., 2015; Prieto et al., 2015). Because the two strategies involve investing in the construction and maintenance of different types of tissues, the trade-off is expected and has even been associated with different successional stages (Paz et al., 2015). Contrary to these studies, our results did not show this dichotomy, and root water storage (RWC) was decoupled from traits related to water and nutrient foraging such as MRD, RMF, and SPRMR. These results mean that both species with deep roots and high investment in root biomass (high MRD and RMF) and species with shallow roots with a high proportion of secondary roots (high SPRMR) can store water in their root tissues. Examples of water storage species with shallow roots are: Sorocea sprucei (Moraceae) and Ruellia macrophylla (Acanthaceae), while Eugenia florida (Myrtaceae) would be the species with deep and water storage roots. Despite this orthogonality, our results show that most of the acquisitive species with water-storage roots had shallow roots with high secondary roots investment. These water-storage roots are associated with a greater capacity for soil exploration, absorption of nutrients and water (Eissenstat et al., 2000; Roumet et al., 2006; Paz et al., 2015; Prieto et al., 2015). Conversely, species with thick and deep roots will have greater resistance to water deficit because these provide more specialized functions for the transport of water and nutrients (Paz et al., 2015; Prieto et al., 2015).
The decoupling between acquisitive-conservative continuum and the roots´ ability to forage water and nutrients could have different explanations. First, as the root system has different functions, mechanical support, and transport and storage of water and nutrients (Eissenstat et al., 2000; Prieto et al., 2015), its functional traits must be optimized to comply with the three functions that could imply the absence of a maximization of a trait associated with a particular function. Second, the water and the essential elements that plants acquire from the soil have different properties, causing the same trait to not be efficient in absorbing all the elements (Weemstra et al., 2016). And third, the physical properties of the soil exert selective forces on the root system, which could limit the maximum development of traits for the acquisition of a specific limiting resource (Weemstra et al., 2016). Under this scenario, it seems likely that the roots have different strategic dimensions and that these vary according to local conditions, which makes it difficult to generate patterns in the correlations between the above and belowground traits.
This study found limited trait coordination between above- and belowground traits and novel trade-offs regarding belowground trait correlation. A remarkable finding was the decoupling between the acquisitive-conservative continuum from the ability to forage water and nutrients of roots. Our results showed that dry forest seedlings have multiple strategies to cope with drought and highlight the importance of incorporating root traits for a comprehensive understanding of the responses of plants to environmental conditions. This variety of functional strategies, and, therefore, the partition of niche between species, could help to explain the high diversity of species at the local scale reported in tropical dry forests. However, it is essential to recognize that traits may vary with ontogeny, which is a variation source rarely accounted. Additionally, these results could help predict the species´ responses to future climatic scenarios, to allow the selection of species capable of establishing themselves under different environmental conditions and guide restoration actions in the most endangered terrestrial ecosystem in the Neotropics.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


