Introduction
Clostridioides difficile is a Gram-positive, spore-forming, anaerobic bacterium that may cause diarrhea in susceptible individuals. It colonizes and proliferates in the gastrointestinal tract of humans in response to structural and functional imbalances in the residing microbiota elicited by antibiotic therapy (Martin et al., 2016). Its main virulence factors are toxins TcdB and TcdA, from the family of large clostridial toxins (Khan & Elzouki, 2014). Additional known virulence factors in this bacterium are cysteine proteases, S-layer proteins, and a binary toxin with ADP-ribosyltransferase activity (Kouhsari et al., 2018).
C. difficile infection (CDI) has increased in terms of severity and incidence in the last two decades (Khan & Elzouki, 2014) and become one of the most critical healthcare-associated diseases in developed and developing countries (Khan & Elzouki, 2014).
C. difficile is a highly heterogeneous species, and so, different strains have emerged and predominated in different places of the world. Of all contemporary strains, the NAP/RT27/ST01 strain (MLST Clade 2) has received the most attention because it includes epidemic and hypervirulent isolates whose infections are more severe and linked to higher mortality rates (O’Connor et al., 2009). Furthermore, it is highly resistant to disinfectants due to the activity of efflux pumps and ABC transporters (Dawson et al., 2011). This genotype has circulated in Costa Rica for more than a decade (Guerrero-Araya et al., 2020). It occasionally predominates, yet it has been outnumbered by other lineages (López-Ureña et al., 2016), such as the NAP9 (MLST Clade 4) and the NAPCR1 (MLST Clade 1) strains.
The NAPCR1/RT012/ST54 strain was detected for the first time during a C. difficile outbreak (Quesada-Gómez et al., 2010) and became endemic in Costa Rican hospitals (López-Ureña et al., 2016). Despite its very small genomic distance to the non-epidemic reference strain CD630 within the C. difficile MLST Clade 1, the NAPCR1 strain shows increased virulence and causes disease by atypical mechanisms (Quesada-Gómez et al., 2015). Subsequent genomic studies have subdivided it into at least three clusters (I, II, and III) owing to a differential representation of mobile genetic elements in its pangenome (Murillo et al., 2018).
C. difficile infection (CDI) is transmitted through the ingestion of endospores, which are characterized by a distinct multilayer ultrastructure and intrinsic resistance to harsh environmental conditions (Paredes-Sabja et al., 2014). The coat layer of endospores is principally responsible for this trait, as it contains highly impermeable disulfide and dityrosine cross-links and detoxifying enzymes, including superoxide dismutase and peroxiredoxin chitinase (CotE). Moreover, the lipids found in the inner membrane of the endospore are significantly compressed by the spore cortex and consequently show low permeability (Leggett et al., 2012; Gil et al., 2017).
CDI patients excrete endospores that can remain viable in the environment for weeks or months until they are ingested by susceptible hosts (Martin et al., 2016). These developmental forms can contaminate bathrooms, wards, beds, and cleaning and medical equipment at the hospital level. Besides, healthcare staff may act as vectors due to poor hygiene practices, such as inadequate handwash or lack thereof (Durovic et al., 2018).
Household bleach (5.25 % sodium hypochlorite) or chlorine-releasing agents, such as sodium dichloroisocyanurate (DCC) (1 000-5 000 ppm), have proven to be more effective than neutral detergents and quaternary ammonium compounds for environmental decontamination of rooms of patients with CDI and high-touch surfaces (Dubberke et al., 2014; Loo, 2015; Turner & Anderson, 2020). When dissolved in water, DCC reaches a pH between 6-7 and increases the availability of free chlorine in the form of hypochlorous acid (Gallandat et al., 2019). This latter compound, as other oxidizing agents, damages the inner membrane of endospores and blocks their germination (Cortezzo et al., 2004).
Given the importance of spores in CDI spreading (Rineh et al., 2014), we hypothesized that the epidemic potential of the NAPCR1 is explained, at least in part, by a greater resistance of its endospores to disinfectants, which has the potential to play important roles in its transmission dynamics. This study was performed to assess this notion and to pinpoint potential explanatory mechanisms based on the availability of whole-genome sequences (WGS) for various NAPCR1 isolates.
Materials and methods
Bacterial strains: This study was done with isolates LIBA-2945 (Cluster I), LIBA-5761 (Cluster II), and LIBA-6276 (Cluster III), which represent each of the three clusters into which the NAPCR1 strain can be classified (Murillo et al., 2018). Strain CD630 and a NAP1 isolate (LIBA-5758) were also studied as a proxy for C. difficile strains with low (Dawson et al., 2011) and high (Ghose, 2013) resistance to disinfectants, respectively.
Preparation of endospores: Bacterial strains were grown at 37 ºC under anaerobic conditions on trypticase soy agar plates supplemented with 0.5 % (w/v) yeast extract. After 5 days of incubation, plates were scratched with sterile loops, and the biomass recovered was suspended in sterile 1X phosphate buffer solution (PBS) containing 1 % bovine serum albumin (BSA, Sigma). These suspensions were homogenized through vigorous vortexing and loaded onto an equal volume of a 100 % (w/v) solution of the non-ionic density gradient medium Histodenzä (Sigma-Aldrich) to reach a final concentration of 50 % (w/v). Endospores were separated from vegetative cells through centrifugation (10 000 rcf x 10 min). Pellets containing the structures of interest were washed thrice with distilled water to remove HistodenzTM remnants (5 000 rcf x 5 min), and the resulting materials were resuspended in 1X PBS supplemented with 1 % BSA to avoid agglutination (Pizarro-Guajardo et al., 2016). The purity and abundance of viable endospores in these preparations were checked by light microscopy and a plate count method. Brain heart infusion agar plates supplemented with 0.01 % (w/v) sodium taurocholate to induce germination were used in this verification step (Roberts & Mullany, 2016). Endospore suspensions were standardized to contain 107 endospores/mL and stored at 4 ºC until use.
Sporicidal assays: The sporicidal activity of 0.1 % (w/v) DCC was determined using a dilution-neutralization method (Vohra & Poxton, 2011). Briefly, a work solution containing 1 000 ppm of DCC prepared from a stock of 10 000 ppm. Later, 100 mL of spores were added to 800 mL of DCC and 100 mL of 1 % BSA to simulate the presence of organic matter in the clinical environment. After 5 min of exposure at room temperature, the activity of DCC was stopped by mixing 100 mL of the mixture with 100 mL of sterile distilled water and 800 mL of 0.5 % sodium thiosulfate as a neutralization agent. Three serial, decimal dilutions of the neutralized mixture were made using 0.85 % sterile physiological saline solution and thereafter 100 µL of each dilution were spread onto Brucella agar plates supplemented with 0.01 % (w/v) sodium taurocholate. After incubation under anaerobiosis at 37 ºC for 72 h, colony counts were recorded and exploited to calculate log10 reduction factors (LRF) (Fraise et al., 2015). A disinfectant agent for clinical use should reach an LRF equal to or greater than 5 to be considered effective (Fraise et al., 2015), which corresponds to a 99.999 % reduction of the initial microbial load. All experiments were performed in triplicate. Differences between disinfectants and strains were examined using one-way ANOVA tests followed by Tukey and Bonferroni post-hoc tests.
Bioinformatic analysis of sporulation genes: Bowtie 2 was used to map trimmed Illumina reads obtained for the LIBA isolates (PRJEB5034, European Nucleotide Archive) to a high-quality, close genome sequence of CD630 (GCF_000009205.2). The resulting bam archives were examined for structural variations in the sequence of genes encoding crucial spore- or sporulation-related proteins (Table 1). Protein sequences were aligned using MUSCLE and the resulting alignments were visualized with Geneious R10.
Table 1 Genes for spore- or sporulation-related proteins scrutinized for structural variants
| Gene (locus) | (Possible) Localization |
| spoIVA (CD2629) | Basement layer a |
| sipL (CD3567) | Basement layer a |
| cotA (CD1613) | Exosporium |
| cotB (CD1511) | Exosporium |
| cotCB (CD0598) | Coat and exosporium a |
| cotD (CD2401) | Exosporium |
| cotE (CD1433) | Exosporium |
| cotF (CD0597) | Exosporium |
| cotJB2 (CD2400) | ND b |
| cotG (CD1567) | Exosporium |
| sodA (CD1631) | Coat |
| bclA1 (CD0332) | Exosporium |
| bclA2 (CD3230) | Exosporium |
| bclA3 (CD3349) | Exosporium |
| cdeC (CD1067) | Exosporium |
a : as yet unconfirmed; b ND: not determined.
Results
Sporicidal effect of disinfectants: As indicated in Fig. 1, DCC fully inactivated spores from strain CD630 (LRF ≥ 5) and induced undistinguishable reductions of intermediate magnitude for the NAPCR1 isolates LIBA-2945 (LRF = 3.64 ± 0.63) and LIBA-6276 (LRF = 3.37 ± 0.46), and the NAP1 isolate LIBA-5758 (LRF = 3.58 ± 0.40). With an LRF that differed by almost two orders of magnitude (1.77 ± 0.47), LIBA-5761 exhibited the highest level of in vitro resistance to DCC overall (Fig. 1). The NAPCR1 isolates were invariably more resistant to DCC than strain CD630 (ANOVA, P < 0.05).
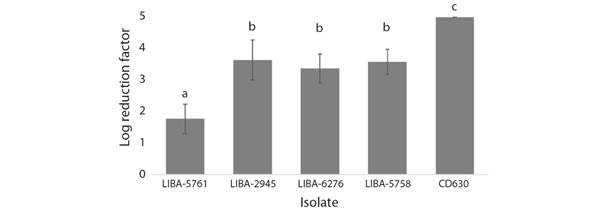
Fig. 1 Activity of DCC against C. difficile endospores. Endospore preparations from three subtypes of the NAPCR1 strain (LIBA-5761, LIBA-2945, LIBA-6276), a NAP1 strain (LIBA-5758), and a reference laboratory strain (CD630), were tested by triplicate using a dilution-neutralization method. Results were expressed as average log10 reduction factors (LRF), whereby an LRF = 5 indicates a 99.999 % reduction in the original number of endospores. A disinfectant should reach this threshold value to be considered effective. Error bars represent standard deviations. Different letters above the bars indicate a statistically significant difference at P < 0.05 (one-way ANOVA).
Bioinformatic analyses: Compared to CD630, all three NAPCR1 isolates exhibited a large deletion in the aminoacid sequence of BclA1. Besides, LIBA-5761 (Cluster II) and LIBA-6276 (Cluster III), showed a deletion of three aminoacid residues in BclA2 (Fig. 2, Fig. 3) No differences were observed in the other analyzed genes.

Fig. 2 Alignment of the predicted BclA1 protein sequences of strain 630 (reference, top) and three subtypes of the NAPCR1 strain. Agreements and deletions to the reference sequence appear masked or as hyphens, respectively. The numbering of the sequence alignment is based on the sequence of strain 630.

Fig. 3 Alignment of the predicted BclA2 protein sequences of strain 630 (reference, top) and three subtypes of the NAPCR1 strain. Agreements and deletions to the reference sequence appear masked or as hyphens, respectively. The numbering of the sequence alignment is based on the sequence of strain 630.
Discussion
This is the first investigation that addresses the response to disinfectants of endospores from the C. difficile NAPCR1 strain. In agreement with our working hypothesis, and possibly in line with its epidemic potential, the NAPCR1 strain was found to be more resistant to DCC than strain CD630. This conclusion was reached by an in vitro experiment; hence it could be strengthened by performing similar tests on surfaces contaminated with different loads and types of organic matter. In most cases, DCC did not reach the LRF ≥ 5 threshold required for a disinfectant agent to be considered effective under our experimental conditions (5 min exposure time, 1 000 ppm). This apparent caveat can be solved by the application of DCC in higher concentrations and for longer contact times (Barbut, 2015). In any case, it is advisable to stimulate the development of alternative compounds on account of the toxicity and corrosiveness of DCC and other chlorine-release agents (Ungurs et al., 2011; Balsells et al., 2016).
This study shows that no disinfection protocol works equally well for all C. difficile strains and even for isolates of the same strain. This finding emphasizes the importance of evaluating multiple field-isolates together with reference strains. Moreover, it justifies continuous typing and testing of the circulating isolates at a given time and place, as they may have been exposed to undetermined selective pressures.
The susceptibility of one NAPCR1 subtype to DCC was unexpectedly low (LIBA-5761, Cluster III). However, this trait could not be linked to a distinct profile of sequence variants in spore- and sporulation-related genes. Of all genes studied, only those for the collagen-like exosporium proteins BclA1 and BclA2 showed sequence polymorphisms. C. difficile has three bclA genes (bclA1, bclA2, and bclA3), and their collagen-like regions are somewhat similar to those found in other sporulated bacteria such as Bacillus cereus and B. anthracis (Pizarro-Guajardo et al., 2014). BclA1 has been characterized to some extent, but even less is known about BclA2 and BclA3.
BclA1 forms stable high molecular mass complexes with other exosporium proteins (Pizarro-Guajardo et al., 2014) and increases the hydrophobicity of the exosporium in B. anthracis (Phetcharaburanin et al., 2014). Based on this knowledge, we predict that the observed deletions in bclA1 and bclA2 lead to the formation of a more robust exosporium layer through increased cross-linking and water repellency, hampering the accessibility of DCC to its internal target in endospores (Cortezzo et al., 2004). This notion could be confirmed through a comprehensive comparison of the composition of the exosporium of strains CD630 and NAPCR1 by electron microscopy and proteomics. On the other hand, since isolates with identical gene deletion profiles displayed different levels of DCC susceptibility, it is plausible that the phenotype of LIBA-5761 is multifactorial and influenced by physiological factors and the activity of efflux pumps or ABC transporters.
Our results highlight the importance of continuously evaluating the efficacy of deployed disinfection agents against circulating strains and hint to a potential role of exosporium proteins in resistance to disinfectants in C. difficile. The relevance of these findings is twofold. On the one hand, it may guide decision-makers and contribute to resource optimization in hospitals. On the other, it deepens our understanding of the functional diversity of C. difficile and its role in disease control.
Ethical statement: The authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that they followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


