Introduction
Rhinella genus (Bufonidae) comprises 94 species distributed in Central (Panama) and South America (Paraguay, Bolivia, Argentina, Uruguay and Brazil), with approximately 30 of them found in Brazil (Pereyra et al., 2016; Wake, 2020). The secretion produced by amphibian paratoid glands is composed by numerous active components such as biogenic amines, peptides, steroids (bufadienolides and bufotoxins), alkaloids (batrachotoxin ‘tetrodotoxin’), and proteins (Gregerman, 1952; Zelnik, 1965; Mahan & Biggers, 1977; O’Rourke, Chen, Hirst, Rao, & Shaw, 2004), comprising the main protection mechanism against potential predators (Clarke, 1997; Kowalski, Marciniak, Rosiński, & Rychlik, 2018). These components, especially those bufadienolides and bufotoxins, have been considered potential therapeutic tools for exhibiting cancer inhibitory activity (Meng et al., 2009), apoptosis suppressive action (Qi et al., 2010) and antimicrobial activity (Tempone et al., 2008). The paratoid gland secretion and their components, e.g., non-enzymatic presynaptic active toxin (730.6 Da) isolated from R. schneideri poison (Rostelato-Ferreira et al., 2018), can induce neurotoxicity characterized by suppression of the motor acetylcholine release (Rostelato-Ferreira, Dal Belo, Cruz-Höfling, Hyslop, & Rodrigues-Simioni, 2011; Rostelato-Ferreira, Dal Belo, Leite, Hyslop, & Rodrigues-Simioni, 2014; Rostelato-Ferreira et al., 2018), including cardiotoxicity consisted in arrhythmia and ventricular failure mostly by antagonizing Na+/K+ATPase of cardiomyocytes (Toledo & Jared, 1995; Sakate & Oliveira, 2000; Gadelha, Lima, Batista, Melo, & Soto-Blanco, 2014; Leal et al., 2020). R. marinus and R. vulgaris are responsible for causing the most cases of envenomation in domestic animals (Sakate & Oliveira, 2000; Sakate & Oliveira, 2001).
In recent years, several biological properties have been characterized from Rhinella schneideri ‘Schneider’s toad’ poison (Rostelato-Ferreira et al., 2011; Rostelato-Ferreira et al., 2014; Abdelfatah, Lu, Schmeda-Hirschmann, & Efferth, 2019; Leal et al., 2020), a species widely distributed in Brazil (Wake, 2020), including the chemical identification and structural characterization of bufadienolides, e.g., marinobufagenin, bufalin, telocinobufagin, hellebrigenin, 20S21R-epoxymarinobufagin and β-sitosterol, which exhibit cytotoxic activity, modulatory action on the complement cascade, anti-inflammatory activity, inhibitory action of serine protease, presynaptic neuromuscular blocking activity and anticonvulsant action (Cunha-Filho et al., 2010; Anjolette et al., 2015; Sousa-Filho et al., 2016; Shibao, Anjolette, Lopes, & Arantes, 2015; Freitas et al., 2017; Rostelato-Ferreira et al., 2018; Baldo et al., 2019; Zheng et al., 2020).
Heparin has been successfully used to neutralize the neurotoxic and myotoxic activities of Bothrops (Viperidae: Crotalinae) ‘pitviper’ snake venoms (Melo & Suarez-Kurtz, 1988a; Melo & Suarez-Kurtz, 1988b; Melo & Ownby, 1999; Calil-Elias, Martinez, & Melo, 2002; Rostelato-Ferreira et al., 2010) and their major phospholipase A2 (PLA2) (Lomonte, Moreno, Tarkowski, Hanson, & Maccarana, 1994a; Lomonte, Tarkowski, Bagge, & Hanson, 1994b; Rodrigues et al., 2004a; Perchuc et al., 2010; Rostelato-Ferreira et al., 2010). The action of heparin against the effects produced by amphibian poisons on the motor neurotransmitter release has not been previously investigated, however, the acid-basic characteristics of steroid derivatives and biogenic amines found in toad venoms may suggest an eventual interaction with heparin (Zelnik, 1965). In addition, acetylcholine (ACh) release modulating drugs, e.g., neostigmine, an acetylcholinesterase inhibitor, and 3.4-diaminopyridine, a voltage-gated potassium channel blocker, show to be effective in counteract the neuromuscular paralysis caused by animal venoms and isolated toxins by promoting the increase of acetylcholine release in motor nerve terminal (Ng et al., 2017; Floriano et al., 2019; Neely, Sabir, & Kohli, 2020). In this work, we have tested different pharmacological strategies in order to counteract the neuromuscular action induced by R. schneideri poison in avian isolated preparation by applying heparin and acetylcholine (ACh) release modulating drugs.
Materials and methods
Reagents and poison: Acetylcholine chloride, neostigmine methylsulfate, 3.4-diaminopyridine and heparin sodium salt were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All salts used to prepare the physiological solutions were of analytical grade. The methanolic extract of R. schneideri poison was provided by Universidade Federal do Pampa (UNIPAMPA, São Gabriel, RS, Brazil) through Dr. Cháriston A. Dal Belo. R. schneideri poison was collected from a single specimen by manual compression from parotid glands and an amount of 2 mg was then included in 50 ml of methanol for three days at room temperature (Gao et al., 2010). The methanolic extract was then lyophilized and maintained under refrigeration until used. Aliquots of the lyophilized extract were dissolved in Krebs solution prior to testing in the neuromuscular preparations.
Animals: This study was approved by an institutional Committee for Ethics in Animal Use (CEUA/UNISO, Protocol no. 096/2016) and the experiments were carried out according to the general ethical guidelines for animal use established by the Brazilian Society of Laboratory Animal Science (SBCAL). Male chicks (4-8 days old, HY- line) were provided by Aviculture Santa Barbara Ltda. (Sorocaba, SP, Brazil) and housed in metal cages with a sawdust substrate at 25 °C on a 12 h light/dark cycle with lights turned on at 6 a.m. The animals had free access to food and water.
Chick biventer cervicis (BC) preparation: Chicks were previously euthanized with isoflurane and the biventer cervicis muscles were removed and mounted under a resting tension of 1 g in a 5 ml organ bath (Panlab, Barcelona, Spain) containing aerated (95 % O2 and 5 % CO2) Krebs solution (composition, in mM: NaCl 118.7, KCl 4.7, CaCl2 1.88, KH2PO4 1.17, MgSO4 1.17, NaHCO3 25.0 and glucose 11.65, pH 7.5) at 37 °C, as described elsewhere (Ginsborg & Warriner, 1960). The preparations were exposed to a field stimulation (0.1 Hz, 0.2 ms, 4-6 V) with stimuli being delivered from a LE 12406 TC stimulator (Panlab, Barcelona, Spain) and the muscle twitches recorded using an MLT0201 force-displacement transducer coupled to a Quad Bridge Amp and PowerLab 4/35 data acquisition system connected to a LabChart Pro v. 6.0 (ADInstruments Pty Ltd., 2020) software (all from ADInstruments Pty Ltd., Sydney, Australia). Muscle contractures to exogenous acetylcholine (ACh, 110 μM) and potassium chloride (KCl, 20 mM) were evoked in the absence of electric stimulation, before and after treatments with poison (10 mg/ml) and heparin (1-30 ml/ml), to assess the function of postsynaptic nicotinic receptors and muscle membrane integrity, respectively (Harvey et al., 1994); the final muscle contractures to ACh and KCl were expressed as a percentage of the resting state, considered as 100 % of response. After the initial assessments with ACh and KCl, the preparations were extensively washed and electrically stimulated for at least 20 min to allow them a stabilization period before treatments. Muscle twitches were recorded for up to 120 min or until complete blockade; some preparations were maintained in Krebs solution alone for 120 min to obtain muscle response control. Other preparations were incubated at 22-24 °C to assess the influence of temperature on poison-induced (10 mg/ml) neuromuscular blockade. Some pharmacological approaches using 3.4-diaminopyridine (91.6 mM) and neostigmine (3.3 mM) were applied to understand the effects of methanolic extract of R. schneideri poison on the avian peripheral neurotransmission. The heparin neutralizing activity on the R. schneideri poison-induced neuromuscular blockade was assessed under two experimental conditions: 1) pre-treatment-poison (10 mg/ml) was previously pre-incubated with heparin (5-150 IU/ml) for 30 min at 37 °C and then tested in the BC preparations; 2) post-treatment-heparin (1 and 30 ml/ml) was added into the recording bath after 10 min exposure to poison (10 mg/ml). The experiments were carried out using four isolated preparations (N= 4) for every protocol described above.
Statistical analysis: The results (myographic recordings) were expressed as the mean ± SEM of four isolated preparations from different animals for each experimental protocol. Changes in the twitch-tension responses of BC preparations were considered as a percentage relative to baseline (time zero) values. Statistical comparisons were performed using Student’s t-test or ANOVA followed by the Tukey-Kramer test, with P < 0.05 indicating significance. All data analyses were done using Origin 8 SR4 v.8.0951 (Microcal Software Inc., 2020) or Prism 5 (GraphPad Software Inc., 2020) software.
Results
Effect of heparin on the R. schneideri poison-induced neuromuscular blockade in chick BC preparation: R. schneideri poison (10 μg/ml) caused complete neuromuscular blockade in indirectly stimulated preparations at 37 °C approximately 70 min after incubation (times for 50 and 90 % blockade: 15 ± 3 min and 4 ± 2 min; P < 0.05 compared to control preparations, N= 5); poison (10 μg/ml)-induced neuromuscular blockade was not prevented by heparin (5 and 150 IU/ml) under pre-treatment experimental conditions (times for 50 and 90 % blockade: 13 ± 2.5 min and 57 ± 3 min (5 IU/ml); 9 ± 1.3 min and 29 ± 2 min (150 IU/ml), respectively; P < 0.05 compared to control preparations, N= 4) (Fig. 1A). There were no important changes in the contractures to exogenous ACh and KCl after incubation with poison (10 μg/ml) alone (106 ± 26 % (ACh) and 120 ± 36 % (KCl) compared to basal; N= 4) or pre-treated with both heparin concentrations (5 and 150 IU/ml) (150 ± 1 (ACh) and 80 ±1 (KCl) for 5 IU/ml; 81.6 ± 1.7 (ACh) and 68.6 ± 11.4 (KCl) for 150 IU/ml, N= 4); these values were expressed as a percentage of the basal contractures, considered as 100 % of response (Fig. 1B).
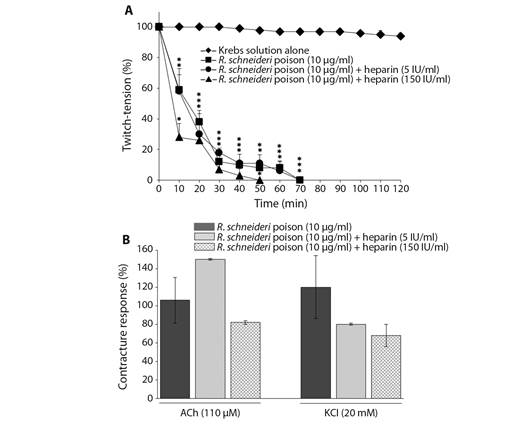
Fig. 1 Neuromuscular blockade induced by R. schneideri (10 µg/ml) poison alone and pre-treated with heparin (5 and 150 IU/ml) in BC preparations. A. Heparin (5 and 150 IU/ml) failure to prevent the poison (10 µg/ml)-induced blockade. B. Unaffected muscle contractures to exogenous ACh and KCl after complete neuromuscular blockade induced by poison alone and pre-treated with heparin. Symbols in section A. and columns in section B. are the mean ± SEM (N = 4). In A. *P < 0.05 compared to control preparations; the superposed (*) represent each point within that time interval. In B. the values were expressed as a percentage of the responses observed in basal contractures recorded before the treatments.
Heparin (5 and 150 IU/ml) has also failed to prevent the R. schneideri poison (10 μg/ml)-induced neuromuscular blockade under post-treatment experimental condition with either the lowest or highest concentrations (times for 50 and 90 % blockade: 10 ± 1.5 min and 46 ± 2.5 min (5 IU/ml); 18 ± 2 min and 50 ± 3 min (150 IU/ml), respectively; P < 0.05, N= 4) (Fig. 2A). The contractures to ACh and KCl were not altered either in those preparations incubated with poison and subjected to post-treatment with 5 and 150 IU of heparin/ml (65 ± 1 (ACh) and 73 ± 1 (KCL) for 5 IU/ml; 112.8 ± 27 (ACh) and 130 ± 30 (KCl) for 150 IU/ml, N = 4; these values were expressed as a percentage of the basal contractures, considered as 100 % of response (Fig. 2B).
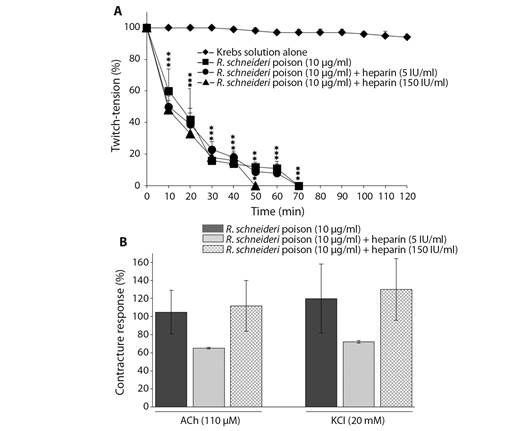
Fig. 2 Neuromuscular blockade induced by R. schneideri (10 µg/ml) poison alone and post-treated with heparin (5 and 150 IU/ml) in BC preparations. A. Heparin (5 and 150 IU/ml) failed to prevent the poison (10 µg/ml)-induced blockade. B. Unaffected muscle contractures to exogenous ACh and KCl after complete neuromuscular blockade induced by poison alone and treated with heparin. The symbols in section A. and columns in section B. are the mean ± SEM (N= 4). In A. *P < 0.05 compared to control preparations; the superposed (*) represent each point within that time interval. In B. the values were expressed as a percentage of the responses observed in basal contractures recorded before the treatments.
Effect of temperature on the R. schneideri poison-induced neuromuscular blockade in chick BC preparation: The poison (10 μg/ml)-induced neuromuscular blockade was abolished in BC preparations maintained at a low-temperature conditions (23-25 °C) for 60 min incubation; however, raising rapidly the temperature to 37 °C after 60 min incubation (without exchanging the physiological media), the poison produced complete neuromuscular blockade which was slightly slower than that seen with BC preparations directly incubated at 37 °C (times for 50 and 90 % blockade: 15 ± 1.4 vs. 23 ± 2# min and 40 ± 3.2 vs. 60 ± 2.5# min for preparations directly incubated at 37 °C and those incubated initially at 23-25 °C and then 37 °C, respectively; P < 0.05 compared to times for 50 and 90 % blockade from those preparations directly incubated at 37 °C, N= 4) (Fig. 3).
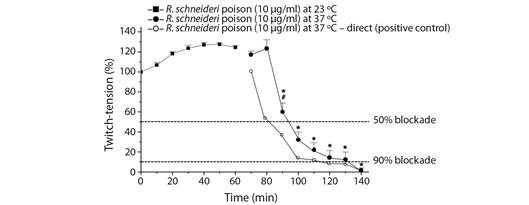
Fig. 3 Influence of temperature on the neuromuscular blockade induced by R. schneideri (10 µg/ml) poison in BC preparations. Incubation at 22-24 °C prevented the poison (10 µg/ml)-induced blockade in indirectly stimulated preparations. The symbols are the mean ± SEM (N= 4). *P < 0.05 compared to the latest point recorded at 22-24 °C; P < 0.05 compared to the time for 50 % blockade from preparations incubated directly at 37 °C.
Effect of neostigmine (NEO) and 3.4-diaminopyridine (3.4-DAP) on the R. schneideri poison-induced neuromuscular blockade in chick BC preparation: In some BC preparations, NEO and 3.4-DAP were tested after complete poison (10 μg/ml)-induced neuromuscular blockade to assess whether they could contribute to reverse the neuromuscular blockade. NEO (3.3 μM) and 3.4-DAP (9.2 μM) did not cause a reversal of the complete poison (10 μg/ml)-induced neuromuscular blockade; however, increasing tenfold the concentration of 3.4-DAP (91.6 μM) there was a partial and sustained reversal of the twitch responses from 10 min incubation (29 ± 7.8 % of maximal reversal reached in approximately 40 min incubation; P < 0.05, N= 4) (Fig. 4).
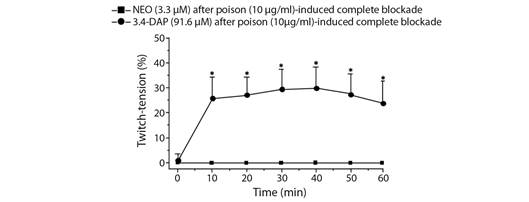
Fig. 4 Effect of neostigmine (NEO) and 3.4-diaminopyridine (3.4-DAP) on the R. schneideri poison-induced neuromuscular blockade by in chick BC preparations. NEO (3.3 µM) failed to reverse the complete blockade induced by R. schneideri poison (10 µg/ml) whereas 3.4-DAP (91.6 µM) produced a partial and sustained reversal of the twitch responses. The symbols are the mean ± SEM (N= 4). *P < 0.05 compared to endpoint after the occurrence of complete neuromuscular blockade.
R. schneideri poison is already known to induce potent blockade of the motor neurotransmission in avian neuromuscular preparations in vitro. The negative modulatory activity induced by R. schneideri poison is preceded by facilitation of the twitch responses, seen with lower concentrations (3 and 10 μg/ml), whilst a highest concentration (30 μg/ml) induces only rapid complete blockade of neuromuscular twitches approximately 20 min, with no evidence of facilitation (Rostelato-Ferreira et al., 2011). Here, we have observed that the methanolic extract of R. schneideri poison (10 μg/ml) produced complete neuromuscular blockade in BC preparations approximately 70 min incubation, with no evidence of facilitation; the reasons for these differences between time for complete neuromuscular blockade and occurrence of facilitation are currently unknown, although they may be attributed to the conditions for extraction and maintenance of the poison and/or its chemical composition due to different amounts of toxic bioactive molecules (Leal et al., 2020).
R. schneideri poison-induced neuromuscular blockade in avian preparations has been associated to a presynaptic mechanism of action, which is characterized by an absent of muscle responses to exogenous ACh and KCl, low creatine kinase (CK) release and no significant morphological damage, indicating that the functionality of postsynaptic nicotinic receptors and muscle membrane integrity were not affected by the poison (Rostelato-Ferreira et al., 2011). Recently, Rostelato-Ferreira et al., (2018) reported the purification and pharmacological characterization of a non-enzymatic presynaptic active toxin (fraction 20) among a total of thirty-two fractions isolated from R. schneideri poison, with molecular mass determined in 730.6 Da; under low concentration, this toxin induces neuromuscular facilitation followed by complete inhibition of muscle twitches recorded in BC preparations (Rostelato-Ferreira et al., 2018), mimicking the neuromuscular effect produced by R. schneideri poison in the same experimental model (Rostelato-Ferreira et al., 2011). The neuromuscular activity of R. schneideri poison has also been investigated in mouse phrenic nerve diaphragm (PND) preparation, in which producing progressive improvement of the acetylcholine release characterized by an increase in the frequency of miniature end-plate potentials and quantal content of evoked end-plate potentials, with no observation of neuromuscular failure (Rostelato-Ferreira et al., 2014).
We also have investigated the efficiency of heparin to prevent the neuromuscular blockade produced by R. schneideri toad poison in BC preparation. Heparin is an acid polysaccharide glycosaminoglycan capable to complex with isolated myotoxins from snake venoms, resulting in neutralization of their toxic activities in vitro. The antitoxic activity of heparin has been validated against the major myotoxins from Bothrops jararacussu (Melo, Homsi-Brandeburgo, Giglio, & Suarez-Kurtz, 1993; Rostelato-Ferreira et al., 2010; Rodrigues et al., 2004a), Bothrops neuwiedi pauloensis (Rodrigues et al., 2004b), Bothrops asper (Lomonte et al., 1994a; Lomonte et al., 1994b), Bothrops moojeni (Perchuc et al., 2010), Crotalus viridis viridis and Agkistrodon contortrix laticinctus (Melo & Ownby, 1999) ‘Viperidae’ venoms. However, there is no previous report about the heparin-induced neutralizing potential to prevent the neuromuscular blockade produced by R. schneideri poison.
Heparin (5 mM) significantly inhibited the cytotoxic activity produced by BnpTX-I, a basic Lys49 phospholipase A2 (PLA2) from Bothrops neuwiedi pauloensis venom, upon C2C12 myoblasts/myotubes (Rodrigues et al., 2004b). Besides, heparin abolished the myotoxic and neurotoxic activities produced by Bothrops jararacussu venom and its major Lys49 PLA2 toxin (BthTX-I) in mouse nerve phrenic-diaphragm preparation (Rostelato-Ferreira et al., 2010) and in chick biventer cervicis preparation (Rodrigues et al., 2004a). Here, heparin did not alter the R. schneideri poison-induced neuromuscular blockade, contrasting with its action against Viperidae venoms and their major toxins (Melo et al., 1993; Lomonte et al., 1994a; Lomonte et al., 1994b; Melo & Ownby, 1999; Rodrigues et al., 2004a; Rostelato-Ferreira et al., 2010). The lack of heparin effectiveness to counteract against the R. schneideri poison-induced neuromuscular blockade may be attributed to its inability to form complex with low molecular weight substances, such as those present in toad poison (730.6 Da) (Rostelato-Ferreira et al., 2018), compared to the molecular weight of PLA2 myotoxins from Viperidae venoms (13-15 kDa).
To examine the role of enzymatic properties in the neuromuscular blockade by R. schneideri poison, experiments in BC preparations were done at 22-24 °C instead of 37 °C. The lower temperature condition is known to attenuate the neuromuscular blockade by Bothrops PLA2 neurotoxins (Cogo et al., 1998; Ponce-Soto et al., 2009; Floriano et al., 2013; Sucasaca-Monzón et al., 2015). Although there is evidence of the presence of PLA2 enzymes in the mucous gland secretions of R. schneideri toad (Shibao et al., 2018), the same was not observed in the poison of this species. However, incubation at lower temperatures abolished the neuromuscular blockade in BC preparations while a mild and progressive neuromuscular facilitation was observed in 60 min exposure time. Furthermore, the R. schneideri poison caused complete neuromuscular blockade immediately after raising rapidly the temperature to 37 °C at the end of incubation (60 min) at low temperature. These findings may suggest that there is an enzymatic influence in the R. schneideri poison-induced neuromuscular blockade. In this context, a PLA2 toxin has been identified in the paratoid gland secretion of a closely related species ‘Bufotes viridis’ (= Bufo viridis) found in Central Asian (Tashmukhamedov et al., 1994).
Neostigmine (NEO), an acetylcholinesterase inhibitor, failed to counteract the neuromuscular blockade induced by R. schneideri poison in BC preparations, suggesting that postsynaptic nicotinic receptors are not involved in the toxic response by toxins present in this poison. However, 3.4-diaminopyridine (3.4-DAP), an antagonist of voltage-gated potassium channels, produced partial and sustained reversal (approximately 30 %) of the neuromuscular blockade by R. schneideri poison in BC preparations. 3.4-DAP antagonizes motor presynaptic potassium channels prolonging the action potential by delaying the membrane repolarization; this effect prolongs the opening of voltage-dependent calcium channels and consequently increase neurotransmitter release (Floriano et al., 2015; Ojala et al., 2021). The mechanism by which 3.4-DAP reverses the R. schneideri poison-induced neuromuscular blockade is unclear. However, since this poison interferes with presynaptic phases to induce neuromuscular failure (Rostelato-Ferreira et al., 2011), the reversal by 3.4-DAP suggests the presence of some sort of presynaptic neurotoxins in the poison that may affect the calcium homeostasis (Rostelato-Ferreira et al., 2018). In addition, bufadienolides present in R. schneideri poison may be directly related to the neuromuscular failure observed in BC preparations by affecting voltage-gated L-type Ca2+ channels (Song et al., 2017).
Taken together, the results presented in this work show that R. schneideri poison induces potent neuromuscular blockade in avian isolated preparation without affecting the functionality of postsynaptic nicotinic receptors or muscle membrane integrity, suggesting the involvement of presynaptic neurotoxins in this response. Heparin failed to prevent the toad poison-induced neuromuscular blockade under pre- and post-incubation treatments whereas neostigmine was not able to reverse the blockade; 3.4-diaminopyridine showed to be partially efficient to reverse the poison-induced neuromuscular blockade, being a potential drug candidate to be used as a pharmacological tool in other physiopathological studies.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


