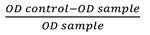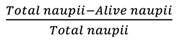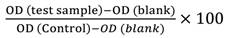Introduction
Natural products have been recognized as the source of medicinal substances and structural sustainability for several years (Beutler, 2019). The natural resources of medicinal plants are precious phytochemicals that are often employed for the treatment of different diseases (Al-Ansari et al., 2019), especially for cancer treatment. Plants as natural resources are used for several years provide potential chemical therapeutics in cancer treatment and interest in nature (Akindele et al., 2015). Hence, phytochemicals cover a wide range of chemical spaces for the discovery of drugs (Mohanraj et al., 2018). Phytochemicals have various pharmacology mechanisms, including stimulating enzymes such as glutathione transferase or preventing cell proliferation (Shareef, Ashraf, & Sarfraz, 2016).
Over a million women with breast cancer are identified per year around the globe (Shareef et. al., 2016); therefore, breast cancer has been the second most common cause of death for women (Azamjah, Soltan-Zadeh, & Zayeri, 2019; Levitsky & Dembitsky, 2014). Since mammography is not available for routine screening, late stages of breast cancer are usually investigated (Shareef et al., 2016). The function of flavonoids in cancer prevention has been documented (Elsyana, Bintang, & Priosoeryanto, 2016). Their ability and healing potential have been separately documented worldwide, indicating that plants could become a prospective source of new medicines (Idris, Wintola, & Afolayan, 2019).
Cassia alata L. (also recognized as Senna alata) is a shrub that belongs to the Fabaceae family (sub-family Caesalpinioideae), which is distributed in the intertropical region (Saito et al., 2012). This plant is popularly known as the candle bush and also ringworm tree due to its folk medicine, which is referenced in the complete flower head (Hennebelle, Weniger, Joseph, Sahpaz, & Bailleul, 2009). It is originally from Central America, primarily found in the Caribbean region, and has also been spread to several tropical climates on all continents (Hennebelle et al., 2009). Senna alata has been utilized primarily for traditional medicine against skin infection, and constipation (Elsyana et al., 2016; Hennebelle et al., 2009) and lately has been suggested for the cosmetic industry as a natural product (Elsyana et al., 2016). Extracts of S. alata are considered to possess antibacterial activity; however, some other antibacterial effects such as prevention bacterial adhesion and biofilm formation besides specific compounds and mechanisms of action are not discovered properly (Saito et al., 2012). This plant possesses potential insecticidal, fungicidal (Iyengar, Rama, & Rao, 1995; Palanichamy & Nagarajan, 1990), anti-inflammatory (Abatan, 1990), antimicrobial (Ibrahim & Osman, 1995; Khan, Kihara, & Omoloso, 2001), wound healing (Palanichamy, Bhaskar, Bakthavathsalam, & Nagarajan, 1991) and antitumor activity (Olarte, Herrera, Villasenor, & Jacinto, 2013; Pamulaparthi & Nanna, 2015; Karchesy, Kelsey, Constantine, & Karchesy, 2016). S. alata leaf extract is traditionally used for treating any type of diseases (Olarte et al., 2013), which is rich in polyphenols and anthraquinones (Fernand et al., 2008). The extensive use of S. alata has been encouraged to look for its pharmaceutically significant compounds in traditional medicine in several research studies (Saito et al., 2012). Traditionally, this plant used for treatment of cancer in Cameroon (mostly breast cancer) (Tene, Tala, Tatong, & Tchuente, 2017). Research on plant chemistry showed that the leaves of S. alata include saponins, anthraquinones, tannins, terpenes, alkaloids, and steroids (Prasenjit, Tanaya, Sumanta, Basudeb, & Kumar, 2016). This significant worldwide herbal medicine has been used historically as an anti-helminthic, anti-inflammatory, uterus illness (Heyde, 1990) and bacterial infection (Igoli, Igwue, & Igoli, 2004; Panda, Padhi, & Mohanty, 2011; Promgool, Pancharoen, & Deachatai, 2014; Prasenjit et al., 2016).
Meyer et al. (1982) identified the brine shrimp lethality bioassay (BSLA) as a particular test that was able to detect screening the range of crude plant extracts in herbal medicine for cytotoxicity in a simple, quick and extensive bioassay for bioactive compounds of natural product (Meyer et al., 1982; Karchesy et al., 2016; Henry, 2017). The brine shrimp lethality test (BSLT) is the primary anticancer test process (Prasetyo, Sidharta, Hartini, & Mursyanti, 2019). However, there is a significant correlation between BSLT toxicity and cytotoxicity in certain cell lines, but this approach is not unique to anticancer activity (Asnaashari et al., 2017). The previous result showed that LC50 value on brine shrimp larvae for ethanol extract of S. alata was 7.7 µg/mL (Logarto, Silva, Guerra, & Iglesias, 2001). The previous study showed that ethanol extract brought more reliable activity than other extracts (Panda et al., 2011). In recent years, GC-MC has developed as a primary technical tool for the secondary profiling of metabolites in both plant and non-plant organisms (Kanthal, Dey, Satyavathi, & Bhojaraju, 2014). Thus, the aim of analysing 80 % ethanolic extract of this study was, therefore, to detect potential chemicals and to separate the compounds and to identify them by GC-MS application (Kanthal et al., 2014). Also, the ethanolic extract of S. alata can cause significant toxic effects on rats due to the presence of some compounds like emodin, aloe-emodin, kaempferol and rhein (Yagi, Tigani, & Adam, 1998). Fernand and colleagues assessed the range of phenolic compounds for S. alata between 81.2 to 106.0 % (Fernand et al., 2008). Therefore, the present study focused on the antioxidant, antiproliferative, and cytotoxicity induced by extract/fractions in breast cancer (MCF-7) cells and normal human mammary epithelial (MCF10A) cells.
Materials and methods
Plant materials: The leaves of S. alata were obtained from Penang Golf Resort (5°31’13.8” N & 100°26’35.4” E) in Bertam (North of Penang State) in November 2019. The plant was identified by a botanist, and a voucher specimen kept at the Herbarium Unit, School of Biological Sciences, Universiti Sains Malaysia, Penang Island, Malaysia.
Extraction Procedure: The leaves (3 Kg) of S. alata were thoroughly washed with double distilled water, dried at room temperature, and pulverized using a mechanical blender (Retsch, ZM200, Germany) at Cluster of Integrative Medicine Laboratory, Advanced Medical and Dental Institute, Universiti Sains Malaysia. Then the powder plants weighed 100 g in each Erlenmeyer flask. Each flask was macerated with hydroalcoholic (80 % ethanol) containing 400 mL of solvent. Maceration was done for three days with mechanical stirring (Bioteck, Elx808) with a constant speed of 150 rpm. The solvent changed daily with a new hydroalcoholic solvent, and residues were macerated in the respective solvent for the next day to reach exhaustive extraction (up to 3 days). After maceration, filtration was performed using Whatman filter paper (150 mm). The rotavapor (Eyela, Japan) was used to concentrate the total filtrate of alcoholic extract to dryness. The concentrated extract was removed from the round bottom flask into a weighed small glass bottle as crude 80 % ethanolic extract. This crude extract was then fractioned by liquid-liquid extraction using separation funnel and resulted in n-hexane, dichloromethane, chloroform, butanol, and aqueous fractions. Vacuum evaporator was used to evaporate each of the extract and fractions. The concentrated extracts were frozen at -2 °C until further application. The yield of each extract was measured and kept until further use. Fifty milligrams of dried samples from maceration, including crude 80 % ethanol extract, n-hexane, dichloromethane (DCM), chloroform, butanol and aqueous were dissolved with 100 % DMSO in 1 mL tube, then sonicated to dissolve the dried samples.
ABTS scavenging activity: The antioxidant activity of various concentration (10, 5, 2.5, 1.25, 0.625, 1563, 0.078 mg/mL) of S. alata 80 % ethanol extract and Trolox was determined by using ABTS assay. ABTS free radical scavenging was carried out as previously explained (Re et al., 1999). Firstly, the stock solutions of 7 mM ABTS solution and 2.45 mM of potassium persulfate solution was prepared and combined to make the working solution ABTS•+ at an 8:12 (v/v) ratio. Then was maintained in the dark at room temperature for 16 to 18 h. The solution was then blended by mixing 4.0-4.5 mL ABTS radical solution with 250 mL distilled water to give an absorbance of 0.70 ± 0.02 at 734 nm. Next, 100 µL extract (0.078 to 10 mg/mL) in absolute ethanol was applied to 180 µL of ABTS•+ working reagent in a 96-well plate. At room temperature, for 45 minutes the 96-well plate was incubated, and the absorbance was recorded at 734 nm. Triplicate tests have been performed. The scavenging capacity was analyzed as a scavenging activity.
Scavenging activity (%)
The percentage of ABTS extract scavenging activity was compared with the percentage of Trolox. A graph of percent inhibition against concentration was used to establish IC50.
Gas chromatographic-mass spectrometry (GC-MS) analysis for crude extract: Elmer Clarus Mass Spectrometer together with the Agilent Gas Chromatography (Santa Clara, CA, U.S.A.) was performed for GC-MS to analyze the 80 % ethanol extract. A 10 µL syringe was used to inject one microliter (1 µL) into the chromatogram system. The Helium gas transported the analyte in the column at a flow rate of 1.2 mL/min. During the examination, a split ratio of 5:1 was performed. Temperature of the injector has been scheduled at 220 °C. The analytes are extracted from capillary column model Agilent 19091S-433 with HP-5MS, 0.25 mm × 30 m × 0.25 film width. Initially the temperature of the oven had been adjusted at 70 °C for 2.00 min, heating up to 280 °C at 10 °C/min. It took 32.5 min overall. The energy used for ionisation was 60.922eV. Mass measurement was conducted at 300 °C. Identification of compounds was achieved by contrasting the mess spectra with the MS library.
Brine shrimp lethality test (BSLT): The cytotoxicity activity of extract and fractions was used using BSLT method. This test was performed following the mentioned protocol by Meyer et al. (1982) and McLaughlin, Rogers, & Anderson (1998) with a bit modification. The larvae of brine shrimp were used as research specimen. Cysts were put and hatched at room temperature for 48 hours with a continuous supply of oxygen, and there is a lamp above the tank’s open side which attracts the hatched shrimps near the wall of the tank, and then incubate for 25-27 °C. The shrimp became matured as nauplii after 48 hours and ready for the experiment. The artificial seawater has been prepared to produce a 38 g/L concentration by dissolving the sea salt, then the unwanted particles were extracted to eliminate them. The number of dead and surviving brine shrimp nauplii was calculated in every well after 6 and 24 hours of incubation under light. Potassium dichromate was dissolved in artificial seawater as a positive control, functioned like a positive control between 0.01 to 3.00 µg/mL concentrations. Larvae from the first day were transferred to the 24-well plates (10 per each well). All the extract and fractions dissolved in saline water and dimethyl sulfoxide (DMSO). As a negative control, a saline media containing DMSO (1 %) were used. Ten nauplii are counted under a dissecting microscope (Meiji Techno, 10X) and then transferred with the aid of Pasteur pipette to each well; for each well a volume of 2 mL has been retained in order to achieve the required concentration for extract. The experiment performed nine concentration of samples (5000, 2500, 1250, 625, 312, 156, 78.1, 39.06, 19.50 µg/mL). Each concentration was conducted in three replicates. When larvae did not show any motion for 10 seconds of monitoring, they were supposedly dead (Meyer et al., 1982). Samples of LC50 (lethal concentration 50 %) higher than 1000 µg/mL is found to be toxic to brine shrimp. The surviving larvae were recorded after 6 and 24 hours of sample exposure. Statistical analysis was used to determine the mortality rate and the lethal concentrations of S. alata extract resulting to 50 % mortality of the brine shrimp (LC50).
Mortality rate (%)
Cytotoxicity using Sulforhodamine B (SRB) assay: Sulphorhodamine B (SRB) assay has been used to determine the cytotoxicity activity of S. alata extracts using breast carcinoma cell line (MCF-7) and normal human mammary epithelial cells (MCF10A) from American Type Culture Collection (ATCC, Manassas, VA, USA). MCF-7 and MCF10A cell lines were cultured in RPMI 1640 and Dulbecco’s Modified Eagle Medium (DMEM) medium, respectively, containing 10 % (v/v) fetal bovine serum (FBS) and 1 % (v/v) penicillin-streptomycin (PS) (Invitrogen Co., Carlsbad, CA, USA). Briefly, 1×104 cells/well of MCF-7 and MCF10A were separately seeded in 96-well plates in a triplicate row and loaded 100 µL culture medium (RPMI 1640 and DMEM, for MCF-7 and MCF10A cell lines, respectively). Microplates were incubated at 37 °C, 5 % CO2, 95 % air, and humidity about 100 %. On the following day, the cells were treated with seven concentrations of extracts (0, 9.38, 18.75, 37.50, 75.00, 150.00, and 300.00 µg/mL) for 24, 48, and 72 hrs. Following these hours, the plate containing extract concentration was incubated, and finally, the test ended by adding cold TCA. 50 µL of cold 30 % (w/v) TCA (at final concentration, 10 % TCA) was applied for in-situ cell fixation with incubation at 4 °C for 30 minutes. Then, the supernatant solution has been discarded, microplates were rinsed with tap water five times and kept for air-dried. Sulforhodamine B (SRB) (50 µL) at 0.4 % (w/v) in 1 % acetic acid was loaded and incubated for 30 minutes at room temperature. Once staining is finished, loose dyes have been retrieved, and the remaining dyes have been removed using five times washing with 1 % acetic acid. After the plates were air-dried at room temperature, and then bounded stain with a 10 mM Tris base. The optical density (OD) of the plate wells has been measured with a microplate reader (Biotek, Elx808) at 570 nm, and the data were held. The percentage survival (viability) of treated cells over the control cells ×100 (T/C) was calculated as cell viability.
% Cell viability
A linear regression of absorbance against the examined concentrations was calculated the concentration at which cell proliferation is inhibited by 50 % (IC50).
Cell imaging: The high-resolution cell microscopes were demonstrated after 72 hours of incubation of cancer cell line and 24 hours for normal cell line, capturing and tracking images using an inverted phase-contrast microscope (Olympus, CKX41) of each concentration for clearly visible cell viability and cell morphology evaluation.
Statistical analysis: Statistical analysis was performed using GraphPad Prism Ver.8 (GraphPad Software, 1996). The means of three replicates are shown in all analytical data (mean ± standard deviation). P ≤ 0.05 was considered statistically significant.
Results
The residue of the plant was then extracted with n-hexane, dichloromethane (DCM), chloroform, butanol and water (aqueous) subsequently in the same way to give 80 % EtOH (10.9 %, yield: 43.9 g), n-hexane (0.22 %, yield: 0.89 g), DCM (0.05 %, yield: 0.18 g), chloroform (0.02 %, yield: 0.09 g), butanol (0.13 %, yield: 0.51 g) and aqueous (0.16 %, yield: 0.65 g) for using 400 g powder leaves of S. alata. This study demonstrates that the ABTS assay IC50 values of Trolox as positive control and 80 % ethanol extract were 0.092 ± 0.02 and 5.59 ± 1.50 mg/mL, respectively (Table 1).
Table 1 Antioxidant activity of 80 % ethanolic extract of S. alata using ABTS assay
| ABTS (Radical scavenging assay) mg/mL | |||
| 80 % Ethanol extract | Trolox | ||
| IC50 value | 5.59 ± 1.50 | 0.092 ± 0.02 | |
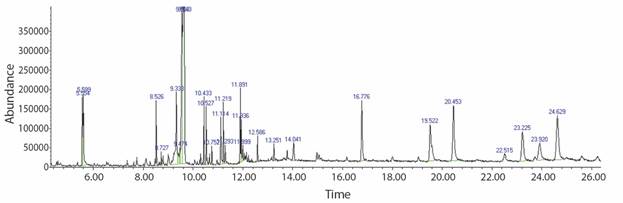
The findings of the GC-MS study of Senna alata ethanolic extract contribute to many compounds being identified. The mass spectrometry attached to the GC classifies these substances. The GC-MS spectrum and the potential cytotoxicity of 80 % ethanol extract (crude extract) to evaluate the biomass chemical groups revealed the existence of various compounds with different retention time, as shown in Fig. 1 and Table 2.
Table 2 Compound investigated in the 80 % ethanol extract of Senna alata in GC-MS
| RT | Name of compound | Molecular formula | Molecular weight (g/mol) | Percentage | Compound nature |
| 5.32 | 1,3,5-Triazine-2,4,6,-triamine | C3H6N6 | 126.11 | 80 | Cyanamide |
| 5.59 | Undecane | C11H24 | 156.31 | 94 | Alkane |
| 7.73 | Phenol,2-propyl- | C9H12O | 136.19 | 87 | Phenylpropanes |
| 8.52 | Cycloheptasiloxane, tetradecamethyl- | C14H42O7Si7 | 519.07 | 91 | Cyclomethicone |
| 8.72 | Ethylparaben | C8H8O3 | 152.15 | 93 | Ester |
| 8.79 | Benzoic acid, 4-ethoxy, ethyl ester | C11H14O3 | 194.23 | 87 | Ester |
| 9.00 | Beta-D-Glucopyranoside, methyl | C7H14O6 | 194.18 | 80 | Glucoside |
| 10.43 | Cyclononasiloxane, octadecamethyl- | C18H54O9Si9 | 667.40 | 91 | Polysiloxane |
| 10.96 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.45 | 93 | Ester |
| 11.11 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 97 | Saturated fatty acid |
| 11.28 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.47 | 93 | Ester |
| 11.88 | Phytol | C20H40O | 296.50 | 90 | Alcohol |
| 12.00 | 9,12,5-octadecatrienoic acid, methyl ester | C19H32O2 | 292.50 | 83 | Ester |
| 12.06 | Octadecanoic acid | C18H36O2 | 284.48 | 95 | Saturated fatty acid |
| 16.76 | Octadecane | C18H38 | 254.50 | 96 | Alkane |
| 16.77 | Eicosane | C20H42 | 282.50 | 98 | Alkane |
| 19.04 | Beta-tocophenol | C28H48O2 | 416.70 | 83 | Tocopherol |
| 19.50 | Eicosane | C20H42 | 282.50 | 91 | Alkane |
| 20.44 | Vitamin E | C29H50O2 | 430.71 | 97 | Tocopherol |
| 24.62 | Gamma.sitosterol | C29H52O2 | 432.70 | 98 | Steroid |
The big fragments of the compound into small compounds lead to peaks with varying ratios of m/z. These mass spectra are the compound fingerprint detectable in the data library.
In this analysis, the formula and structure of 20 biomolecules can be predicted. Further study can proceed to the isolation of bioactive compounds, and their structural clarification and evaluation and screening of pharmaceutical activity will be useful for further drug research. GC-MS investigated steroids (ɣ-sitosterol), linear alkanes (undecane, octadecane, eicosane), esters (ethylparaben, benzoic acid, hexadecanoic acid, ethyl ester, hexadecanoic acid, methyl ester), tocopherol (vitamin E and β-tocopherol) as well as fatty acid such as n-hexadecanoic acid and octadecanoic acid in the 80 % EtOH extract.
Only two hours after an interaction with the higher potassium dichromate concentration, there was a fatal effect in the brine shrimp. The LC50 value for potassium dichromate was 43.76 μg/mL for the corresponding regression line and showed toxic signs (LC50 against the brine shrimp was less than 1 000 µg/mL). Due to high toxicity on A. salina cysts, potassium dichromate has shown limited hatching success. The median lethal concentration of the brine shrimp lethality assay (LC50) for Senna alata leaf extract/fractions are shown in Table 3.
Table 3 Cytotoxicity activity of various extracts of Senna alata on brine shrimp
| Extract/fraction | LD50 (µg/mL) | |
| 6 hrs (acute) | 24 hrs (chronic) | |
| 80 % EtOH (Crude extract) | ND | ND |
| Hexane | ND | ND |
| Dichloromethane | ND | 1 432 |
| Chloroform | 2 520 | 1 214 |
| Butanol | 1 447 | 1 034 |
| Aqueous | 5 053 | 2 428 |
ND = not determined; LD50 value for potassium dichromate was 43.76 µg/mL.
Note: The brine shrimp mortality percentage were measured as mean ± SD.
Of the six extracts tested, 2 exhibited no toxicity to the brine shrimps. These included 80 % ethanolic crude extract and n-hexane fraction, in which no mortality was observed during screening. Dichloromethane, chloroform, butanol, and aqueous fractions showed an LC50 value higher than 1 000 µg/mL. There was no cytotoxic effect on any of the concentrations of the candle bush 80 % ethanol extract and hexane fraction using the BSLT method and, brine shrimp were still moved vigorously. However, the other four extracts showed practically non-toxic (LC50 > 1 000 µg/mL) to brine shrimps. These extracts were aqueous, dichloromethane, chloroform, and butanol with LC50 values between 1 034-2 428 µg/mL (after 24 hrs).
In the present study, the cytotoxic effect (IC50) of the crude ethanol and fractioned extracts (hexane, dichloromethane, chloroform, butanol and aqueous) were identified on one human cancer cells (MCF-7) and one normal non-cancer cells (MCF10A) using the SRB assay. 80 % EtOH extract did not show toxicity on both cell lines (Fig. 2A). Hexane fraction of S. alata exhibited an excellent inhibition towards MCF-7 cells with IC50 of 0.013 µg/mL at 72 h, in comparison to IC50 values of 48 h (Fig. 2B). It is interesting to note that this fraction did not show cytotoxicity against MCF-7 cells at 24 hours. Others, such as dichloromethane and butanol against MCF-7 cell line after 72 hours with IC50 values of 47.11 and 57.61 µg/mL, respectively, have been shown to have significant cytotoxic activity (Table 2; Fig. 2B, Fig. 2C, Fig. 2E). Generally, the 80 % EtOH, chloroform, and aqueous exhibited weaker cytotoxicity profile against the MCF-7 cell line (IC50 > 100 µg/mL). The viability of untreated control cells corresponds to 100 % because all extracts had no cytotoxic effect on the normal cell, though we did not analyze the selectivity index (SI). Although, most importantly, all the extracts did not show the cytotoxic effect on MCF10A as normal human mammary epithelial cells. In addition, IC50 values were determined for SRB assay, extracts and the results are tabulated (Table 4), and also in Fig. 2.
Table 4 Inhibition concentration (IC50) of various extracts of Senna alata against breast cancer (MCF-7) and normal human mammary epithelial cells (MCF10A)
| Extract/fraction | MCF-7 | MCF10A | ||
| IC50 (µg/mL) | ||||
| 24 hrs | 48 hrs | 72 hrs | 24 hrs | |
| 80 % EtOH | > 100 | > 100 | > 100 | ND |
| Hexane | ND | 9.626 | 0.013 | ND |
| Dichloromethane | > 100 | 60.03 | 47.11 | ND |
| Chloroform | > 100 | > 100 | > 100 | ND |
| Butanol | 89.30 | 41.98 | 57.61 | ND |
| Aqueous | > 100 | > 100 | > 100 | ND |
ND = not detected.
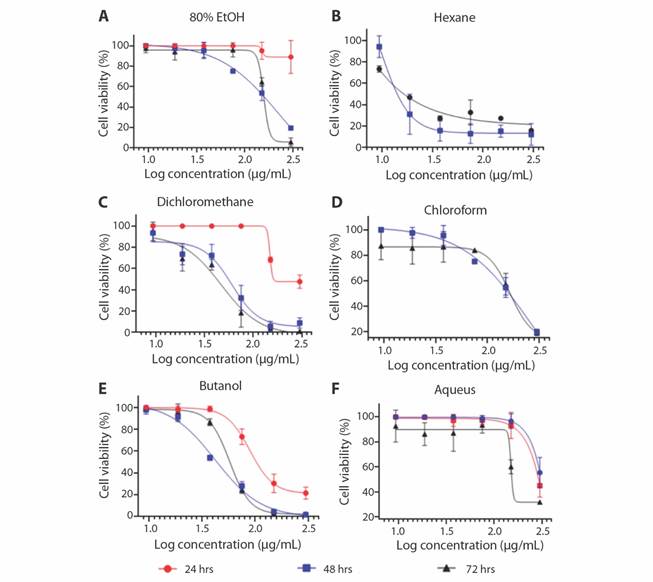
Fig. 2 In vitro cytotoxic activity of various extracts in MCF-7 cells (Human breast cancer cells) by SRB assay at different times of exposure (24, 48 and 72 hours). All the values are mean ± SD of three samples. A. 80 % Ethanolic extract, B. Hexane, C. Dichloromethane, D. Chloroform, E. Butanol, F. Aqueous fraction.
Treated cells were observed for the morphological feature using a bright-field microscope (Olympus, CKX41) at 4X and 10X magnification. MCF-7 and MCF10A cells treated with various extract/fractions and then observed after 72 h incubation (Fig. 3). The results only showed for hexane (Fig. 3A, Fig. 3D, Fig. 3G), DCM (Fig. 3B, Fig. 3E, Fig. 3H) and butanol (Fig. 3C, Fig. 3F, Fig. 3I) fractions.
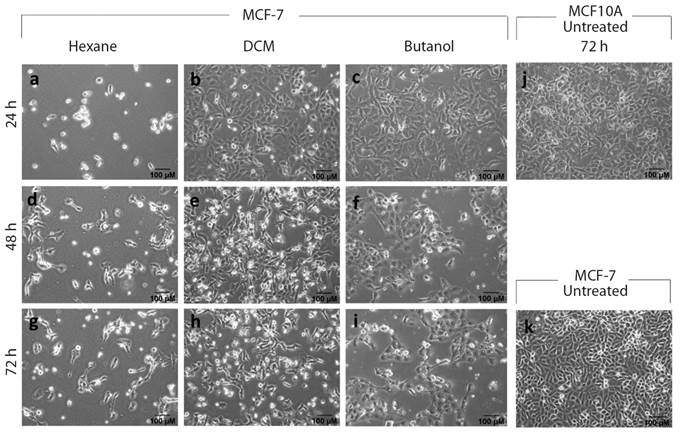
Fig. 3 Morphological changes of MCF-7 and MCF10A cells treated with extract/fractions of Senna alata L. during 24, 48 and 72 h. IC50 calculated with the SRB assay evaluating dose-responsive curves. Various cell forms shown on MCF-7 and MCF10A, treated with S. alata during 72 h. Vehicle DMSO is used to treat control cells. A. MCF-7 cells with hexane treatment at 24 hrs; B. Cells with dichloromethane treatment at 24 hrs; C. MCF-7 cells with butanol treatment at 24 hrs; D. MCF-7 cells with hexane treatment at 48 hrs; E. MCF-7 cells with dichloromethane treatment at 48 hrs; F. MCF-7 cells with butanol treatment at 48 hrs; G. MCF-7 cells with hexane treatment at 72 hrs; H. MCF-7 cells with dichloromethane treatment at 72 hrs; I. MCF-7 cells with butanol treatment at 72 hrs; J. MCF10A cells untreated as control after 72 hrs; and K. MCF-7 cells untreated as a control after 72 hrs.
Significant phenotypic differences were observed in the presence of Senna alata extracts as cancer cell line was incubated (Fig. 3). From cell photographs at first day (24 hours) that the cells treated with fractions in Fig. 3B and Fig. 3C the cells and their volume started to decrease and round shape in contrast to the control of the MCF-7 cells treated with Tamoxifen which were a simple function of apoptosis (figure not shown). After 48 and 72 hours, cells became cluster together, exhibited membrane blebbing (Fig. 3D, Fig. 3E, Fig. 3F, 48 hrs), and began to detach from the dish (Fig. 3H, Fig. 3I, 72 hrs). Normal MCF10A cells, by contrast, have not shown those significant morphologic changes (data not shown). This indicates that S. alata is effective and reasonably non-toxic for folk/conventional drugs and appropriate for cancer treatment.
Discussion
Breast cancer is the world’s second most fatal illness for women (Kamalanathan & Natarajan, 2018). Several other findings have shown that numerous medicinal plants can be used to prevent the growth of human breast cancer (Kamalanathan & Natarajan, 2018). However, a collection of antioxidant compounds exists in herbs, fruits and plants have already shown that breast cancer cells are destroyed by them without no toxic effect on normal cells (Raj, Ireland, Ouhtit, Gaur, & Abdraboh, 2015). Both BSLT and ABTS (antioxidant assay) are easy to handle, low cost, and use small quantities of test equipment (Peteros & Uy, 2010; Asnaashari et al., 2017). The ABTS radical-scavenging measuring technique, a popular method utilized to test the antioxidant activity, gains from adopting a hydrogen ion from the antioxidant, decolorizing its blue colors, as ABTS free radicals become steady (Lee, Oh, Cho, & Ma, 2015). The ABTS assay seems to be more sensitive than DPPH assay in detecting antioxidant activity due to extreme faster reaction kinetics, and its reaction to antioxidants is stronger (Lee et al., 2015), and The ABTS radical is significantly more water-soluble than DPPH (He et al., 2010). Although the antioxidant activity of leaf extract from S. alata fractionation obtained a new indole alkaloid, 1-(4′-hydroxyphenyl)-2,4,6-trihydroxy-indole-3-carboxylic acid that exhibited strong antioxidant potential with an IC50 of 0.0311 μM ± 0.002 (Olarte, Herrera, Villasenor, & Jacinto, 2010). In other study, ethanol extract from leaves of this plant showed 67% of the antioxidant activity (Sagnia et al., 2014). Also, the hexane extract of S. alata showed no free radical scavenging activity (Jacinto, Olarte, Galvez, Villasenor, & Pezzuto, 2005). To identify bioactive compounds from 80 % ethanolic extract of S. alata, our GC-MS result confirmed the study by Ali et al. (2017), which found the same compounds mostly, fatty acids composition from leaves of Senna alata (Ali et al., 2017).
It indicated that the brine shrimp lethality test was helpful in assessing the toxicity of the plant extract (Sahgal et al., 2010). This procedure involves exposure of brine shrimp larvae to plant extract in saline media, and the death of larvae is measured after one day (Mayilsamy & Geetharamanan, 2016). Logarto has shown that a strong link was found between the LC50 of the brine shrimp lethality test and LD50 in the acute oral toxicity test in mice (r = 0.85;P < 0.05) (Logarto et al., 2001). Upon 24 hours of treatment, Artemia salina larvae with LC50; if the sample extract is LC50 < 1 000 μg/mL, its toxicity is high, and the cytotoxicity is expected to occur. The level of toxicity would have an anticancer effect on extracts (Prasetyo et al., 2019). Evaluating the efficiency of hatching cysts concerning the time of exposure showed that extracts had notable hatching success after 36-48 hours, which would be the greatest hatching time for brine shrimp (Meyer et al., 1982; Braguini, Pires, & Alves, 2018).
The method of Meyer et al., graded as toxic (LC50 value < 1 000 μg/mL) and non-toxic (LC50 value > 1 000 μg/mL) for crude extracts and pure materials (Meyer et al., 1982; Naher et al., 2019). Another study revealed that seed extract showed more toxic than leaf extract of S. alata showed LC50 value at 4.31 and 5.29 ppm, respectively, from the result of the brine shrimp lethality test (Rahman, 2004). Also, the LC50 value of the C. alata seed oil extract was at 250 µg/mL (Mannan et al., 2011), and 7.74 µg/mL (Parra, Yhebra, Sardiñas, & Buela, 2001). This suggests that these fractions may contain no cytotoxic compound. Brine shrimp mortality was predicted to be related to bioactive compounds and not malnutrition after exposure to dichloromethane, chloroform, butanol, and aqueous fractions. However, the percentage of deaths as time and concentration was increased for these fractions and the existence of toxic compounds in the fractions, which requires further examination, may lead to that effect. Several studies showed a strong correlation with different tumor cell lines in the BSLT (Elsyana et al., 2016). In BSLT, the cytotoxicity activity of the extract is determined by a 50 % death response to brine shrimp (LC50) (Elsyana et al., 2016). Based on our hexane fraction results from MCF-7 cell line, and according to Elsyana et al., compared this fraction with other extracts and fractions containing flavonoids and triterpenoids, the maximum cytotoxic activity was reported by hexane fraction (Elsyana et al., 2016). Also, Olarte and colleagues (Olarte et al., 2013) found out that the hexane extract from S. alata showed the highest growth inhibition against MCF-7 cell line among three other extracts with IC50 value 16 µg/mL which confirm our present study with IC50 values 9.63 and 0.01 µg/mL for 48 and 72 hrs, respectively. However, based on the National Cancer Institute guideline (NCI, USA) that 30 µg/mL is the higher IC50 ranges assumed reasonable for purification of an extract (Akindele et al., 2015). Another study revealed that hexane fraction of S. alata possessed cytotoxic effect against lung cancer cell (A549) and ovarian cancer cells (OV2008) (Levy & Carley, 2012). Also, ethyl acetate extract of S. alata by other studies showed 50 % inhibition (GI50) value at 5.90 µg/mL against the MCF-7 cell line (Onyegeme-Okerenta, 2018). On the other hand, the chloroform fraction showed anticancer activity against MCF-7 with IC50 value 37.4 µg/ mL (Ali et al., 2017). According to other studies, chloroform extract from the stem of three species from Cassia sp., namely, C. glauca, C. obtusifolia and C. sophera showed high cytotoxicity against MCF-7 cell line (Shankar & Surekha, 2017). Emodin was previously separated from S. alata leaves (Prasenjit et al., 2016; Ali et al., 2017) and showed anticancer activity (Hsu & Chung, 2012). These findings revealed that there is a direct connection between the brine shrimp lethality test and in vitro cytotoxicity towards the S. alata extracts. In the present study, we displayed that hexane and butanol fractions induce apoptosis in MCF-7 human breast cells in a time- and concentration-dependent basis, which is similar with previous studies using different extracts of S. alata (Olarte et al., 2013; Onyegeme-Okerenta, 2018). Our finding indicates that S. alata extracts cytotoxicity is performed through apoptotic cell death in tumor cells. In a study by Olarte and colleagues that they treated hexane fraction with MCF-7 cell line. The MCF-7 cells rounded up and missed contact with adjacent cells between 12-24 hrs (Olarte et al., 2013).
The anticancer function of flavonoids and triterpenoids, according to their antioxidant characteristics, is consistent with their capacity to scavenge free radicals, to suppress radical oxygen species (ROS), enzymes and to prevent cells and extracellular compound oxidation (Elsyana et al., 2016). Flavonoids and triterpenoids were concentration-dependent toxic to the brine shrimp and, therefore, could have resulted in the death of brine shrimp (Elsyana et al., 2016). Several studies have shown that flavonoids can prevent the proliferation and delay of tumor cells (Razak et al., 2019). Assessment of bioactive compounds such as flavonoids, alkaloids, glycosides, carbohydrates, protein, saponins, triterpenoids, and amino acids indicated the existence of most of the component in polar extracts such as ethanol, methanol and aqueous extracts comparison with nonpolar extracts such as petroleum ether and chloroform. Though, all extracts possessed flavonoids, phenols, and tannins (Panda et al., 2011). Because of its perfect fundamental chemistry to free radical scavenging activities, phenols are a significant class of antioxidants (Chaudhary et al., 2015). However, S. alata extracts showed potential cancer cell inhibition and reduced the risk of further proliferation based on the results of the SRB assay. Jacinto et al. (2005) identified a high cancer chemo preventive ability while S. alata hexane leaf extract was found to cause a particular activity of the quinone reductase similar to the bromoflavone as a chemo preventive agent (Jacinto et al., 2005). More pharmacological and phytochemical tests are worthwhile in this research to establish the exact principal cytotoxicity compound reaction.
The result of this study shows S. alata could be an outstanding lead in the progress of breast cancer anticancer agents (IC50 < 100 µg/mL), which did not exhibit toxicity on normal cell line as well. Interestingly, in contrast to SRB assay results, the S. alata extract/fractions exhibited non-toxic activity (LC50 > 1 000 µg/mL) was assessing using the brine shrimp lethality test as a primary assay for anticancer activity. The source of organic antioxidants is available and provides significant medical benefits. It could be inferred from GC-MS findings that S. alata contains many bioactive compounds. Our laboratory is also investigating further research to clarify the mechanism of action of apoptosis in breast cancer and bioactive compounds, which will be published in a future manuscript.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio