Introduction
Coral reef ecosystems grow in relatively poor waters, however, are areas of high productivity and abundance of organisms. Within the coral reef ecosystem, there is a tight recycling of nutrients and a self-generated food web (Porter & Porter, 1977). Zooplankton is a major link in the food webs of coral reefs (Alldrege & King, 2009) and a key source of allochthonous nutrients for the corals (Yahel, Yahel, & Genin, 2005a). The coral reef zooplankton is represented by complex assemblies from different sources: resident species, demersal migratory species, holozooplankton species from oceanic waters transported onto the reef, and reef merozooplankton species (Heidelberg, Sebens, & Purcell,2004). This complex diversity provides substantial nutrient inputs for coral reef fishes, corals and other predators (Heidelberg, O’neil, Bythell, & Sebens, 2010), bringing inorganic nutrients and vitamins which cannot be obtained by the corals through zooxanthellae photosynthesis.
The diel migration patterns of the coral reef zooplankton are known to increase both the biomass and abundance at night, thus becoming an important energy source for plankton feeders like corals (Nakajima, Yoshida, Othman, & Toda,2008; Nakajima, Yoshida, Othman, & Toda,2009). Nocturnal migration in coral reefs is considered as a mechanism to avoid the high predation that occurs near the bottom by zooplanktivores such as corals (Sebens, Grace, Helmuth, Maney, & Miles, 1998), and is suggested that these migrations are one of the major causes shaping the temporal variations of community structure and abundances of zooplankton over coral reefs (Yahel, Yahel, Berman, Jaffe, & Genin, 2005b).
Characterize the community structure over coral reef surfaces could be a challenging task, due to the artifacts of different sampling strategies, topographically complex structures and changes in zooplankton composition due to diel migration (Heidelberg et al., 2010). One of the first attempts to quantify, describe and register the diel migration of the zooplankton community over coral reefs was made by Emery (1968) in the Florida Keys, using nets and a suction device at different times of the day, nineteen taxonomic groups were identified, being copepods the most abundant. Alldredge and King (1977), using emergence traps placed over different substrates, quantified demersal zooplankton at Lizard Lagoon in the Great Barrier Reef. They found a significant preference of substrate by demersal zooplankton and a distinct pattern of nocturnal vertical migration. More recent studies using traps and other devices have recorded the diel migration pattern over coral reefs (Yahel et al. 2005b; Nakajima et al. 2008; Alldredge & King, 2009; Nakajima et al., 2009), registering zooplankton abundance peaks at dusk. Recently, Andradi-Brown compared shallow and mesophotic zooplankton communities at Utila island in the Mesoamerican Barrier Reef, and Smith, Richter, Fabricius, and Cornils (2019) described the substrates preferences and migration behavior of Labidocera spp. in two coral reefs systems in Papua New Guinea. Despite these efforts characterizing the community structure of zooplankton over coral reefs substrates, information about reef-associated demersal and pelagic zooplankton in oceanic islands is very scared. Several zooplankton studies at Isla del Coco have described different taxonomic groups (Jiménez-Cueto, Suárez-Morales, & Morales-Ramírez,2012; Gasca & Morales-Ramírez, 2012; Castellanos, Hernández, Morales-Ramírez, & Corrales,2012), new species (Suárez-Morales & Morales-Ramírez, 2009; Suárez-Morales & Gasca, 2012; Esquivel-Garrote, Suárez-Morales, & Morales-Ramírez, 2015) and the zooplankton community; however, no studies have been made to characterize the zooplankton community over coral reef substrates.
Due to lack of information about zooplankton associated to different coral reef substrates in oceanic islands, the objectives of this study were to characterize the community structure of the zooplankton associated to different coral reefs substrates, to establish the nocturnal variation of the zooplankton community according to substrate type or/and sampling hour and to increase the knowledge of the benthic copepod fauna over different substrates in a coral reef system in Chatham Bay at Isla del Coco National Park, an oceanic island in the Eastern Tropical Pacific.
Materials and methods
Study site: Isla del Coco is an oceanic island located 530 km south-west from the Pacific coast of Costa Rica, in the Eastern Tropical Pacific (ETP) (5º32’N-87º04’W). It has 24 km2 of land and 1 997 km2 of marine surface. In 1978 it was declared a National Park and UNESCO deemed it as a Human Heritage Site in 1997 (Cortés, 2008). The island is influenced by the North Equatorial Countercurrent (NECC), with a seasonal variation in its intensity (Lizano, 2008). The west-east flow carries water and plankton from the central Pacific Ocean to the American coast; thus, Isla del Coco is primarily affected by the NECC and is the first point for species establishment and distribution in the ETP (Cortés, 2008). Samples were collected in a coral reef located in Chatham Bay (Fig. 1), characterized by a high degree of rugosity and covered mainly by sand. Coral cover is moderate and dominated by Porites lobata, and there is low coverage of crustose calcareous algae.
Sampling: The sampling was conducted during the night of July 3, and the dawn of July 4, 2011. One zooplankton trap was placed over four different kinds of substrates (5-10 m deep): big P. lobata colonies (BPC), small P. lobata colonies (SPC), dead and live coral (DaAC, different from P. lobata), and sand. The traps consisted of a cone made with plastic bags, and a collector cup (mesh size: 100 μm) attached at the end of the aperture; chains were used at the base of the traps to fix them to the substrate (Fig. 2). The traps were placed at 1800 h and the collector cups were collected at 2100, 0000 and 0600 h. Samples were fixed using 4% formaldehyde. A total of 12 samples were collected, one per each time interval, over the four different substrates. The samples were analyzed at the Zooplankton Laboratory (CIMAR, UCR). The total of organisms per sample was counted and identified to the minimum taxonomical possible level, focusing mainly on the species-level identification of copepods. The amount of individuals was expressed as abundance.

Fig. 2 Zooplankton traps used to collect samples in different substrates and different time intervals at Isla del Coco, during July 2011. Photo: By Jaime Nivia.
Statistical analysis: Totalabundances of zooplankton were transformed using log X+1. An ANOSIM was conducted to test similarities of zooplankton composition among substrates and sampling time intervals. A SIMPER test was used to determine the contribution of each taxon to dissimilarities in the community structure.
Results
A total of 5 049 individuals were counted from 22 different taxonomic groups, corresponding to 90 taxa (i.e. putative species). The copepods were the dominant group in all samples. Twenty new records of families, genera, and species of benthic copepods were found for Costa Rican waters: one genus of the family Cyclopinidae and Clausidiidae (Order Cyclopoida), and seven families, eight genera and ten species within the Order Harpacticoida (Table 1). Fifty-three copepod species were identified, the order Harpacticoida was the richest with 20 species (n=706 individuals; 76 benthic, 630 planktic), then Calanoida with 14 species (n=280), and Cyclopoida (8 spp., n=482). Planktic copepods were most abundant. The harpacticoid Microsetella rosea was the most abundant copepod species (32%), followed by Siphonostomatoida and the cyclopoids Oncaea spp., which reached 23% and 14% of the total abundance, respectively.
TABLE 1 List of benthic copepods collected in traps place in different substrates of a coral reef (5-10 m depth) in Isla del Coco National Park, Costa Rica
| Order | Family | Genera | Specie |
| Cyclopoida | Clausidiidae | Hyphalion** | sp. |
| Cyclopinidae* | Cyclopuella** | sp. | |
| Harpacticoida | Ameiridae* | indet. | sp. |
| Dactylopusidae* | Dactylopodopsis cf. | dilatata*** | |
| Dactylopusia** | sp.1 | ||
| Dactylopusia** | sp.2 | ||
| Harpacticidae | Harpacticus cf. | obscurus*** | |
| Mucropedia** | sp. | ||
| Idyanthidae* | Idyellopsis cf. | typica*** | |
| Laophontopsidae* | Aculeopsis cf. | longisetosa*** | |
| Miracidae | Teissierella cf. | salamboi*** | |
| Parastenheliidae* | Parastenhelia** | sp. | |
| Peltidiidae | Alteuthella** | sp. | |
| Peltidiidae Porcellidiidae* | Parapeltidium** | sp. | |
| Porcellidium cf. | yoroium*** | ||
| Thalestridae | Eudactylopus cf. | krusadensis*** | |
| lucayosi*** | |||
| Eudactylopus** | sp. | ||
| Parathalestris cf. | incerta*** | ||
| Tisbidae* | Tisbe** | sp. |
*New family record, ** new genera record, *** new species record for Costa Rica and Isla del Coco.
Copepods nauplii (42%) and adult copepods (38%) were the most dominant taxa in all time intervals, while appendicularians and decapod larvae were the most abundant taxa following copepods (4% and 2%, respectively) (Table 2). The abundance between time intervals showed dissimilarities among community composition (ANOSIM, R=33.60%, p=0.003). Adult copepods were abundant in every substrate except in P. lobata colonies between 0000 h and 0600 h, and nauplii reached higher abundances from 1800 to 2100 h. Copepodites were more abundant from 2100 to 0000 h in small P. lobata colonies (Fig. 2F), and decapod larvae from 1800-2100 over the sand patches. Pterotracheoidea were more abundant in small and big P. lobata colonies and in dead and live corals between 1800 and 2100 h. The highest amount of individuals was recorded between 0000 and 0600 h in every substrate (Fig. 3).
TABLE 2 Relative abundance of each taxonomic group of coral reef zooplankton during different time intervals between dusk and dawn, Chattam Bay, Isla del Coco National Park
| Taxa/Time interval | 1800-2100 | 2100-0000 | 0000-0600 | Total abundance |
| Adult copepods | 23,9 | 27,6 | 49,7 | 37,9 |
| Nauplii | 50,1 | 45,2 | 35,1 | 41,6 |
| Copepodite | 5,0 | 15,4 | 7,1 | 8,1 |
| Appendicularia | 4,6 | 5,7 | 2,6 | 3,9 |
| Isopoda | 1,0 | 1,9 | 1,6 | 2,1 |
| Amphipoda | 0,2 | 2,3 | 1,1 | 1,5 |
| Polychaeta | 0,5 | 0,2 | 0,6 | 1,5 |
| Mysidacea | 1,3 | 0,8 | 0,4 | 1,2 |
| Chaetognatha | 0,1 | 0,2 | 0,4 | 0,8 |
| larva decápodo | 7,1 | 0,3 | 0,3 | 0,5 |
| Tanaidacea | 0,3 | 0,1 | 0,2 | 0,2 |
| Pteropoda | 0,0 | 0,0 | 0,2 | 0,2 |
| Ictioplancton | 0,0 | 0,0 | 0,1 | 0,1 |
| Heteropoda | 5,6 | 0,0 | 0,1 | 0,1 |
| Stomatopoda | 0,1 | 0,0 | 0,1 | 0,1 |
| Euphausiacea | 0,0 | 0,1 | 0,1 | 0,1 |
| Cipridina | 0,2 | 0,0 | 0,1 | 0,1 |
| Salpida | 0,0 | 0,0 | 0,0 | 0,1 |
| Ostracoda | 0,0 | 0,0 | 0,0 | 0,0 |
| Cnidaria | 0,2 | 0,0 | 0,0 | 0,0 |
| Doliolida | 0,0 | 0,1 | 0,0 | 0,0 |
| Cephalopoda | 0,0 | 0,1 | 0,0 | 0,0 |
| Total % | 100 | 100 | 100 | 100 |
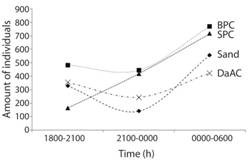
Fig. 3 Abundance of zooplankton (N=5049) in the traps at different time intervals per substrate: big P. lobata colonies (BPC); small P. lobata colonies (SPC); dead and other alive corals (DaAC), and sand.
Benthic copepods (harpacticoids, and cyclopoids), were more diverse from 0000 to 0600 h, comprising 18 taxa. However, the highest amount of individuals was registered between 1800-2100 h. The benthic harpacticoids were the most abundant in each time of sampling. Copepods showed a variation in abundance among time intervals, Microsetella rosea was the most abundant in every substrate between 0000-0600 h (Fig. 4A, Fig. 4C, Fig. 4E), except in dead and alive corals where Siphonostomatoida were more abundant (Fig. 4G); Paracalanus aculeatus also showed higher abundances between 0000-0600 h (Fig. 4B, Fig. 4D, Fig. 4H), while individuals of Siphonostomatoida were present in every substrate with the highest abundances registered from 0000 to 0600 h (Fig. 4A, Fig. 4C, Fig. 4F, Fig. 4G). In general, the higher relative abundance (48%) was recorded from 0000 to 0600 h.
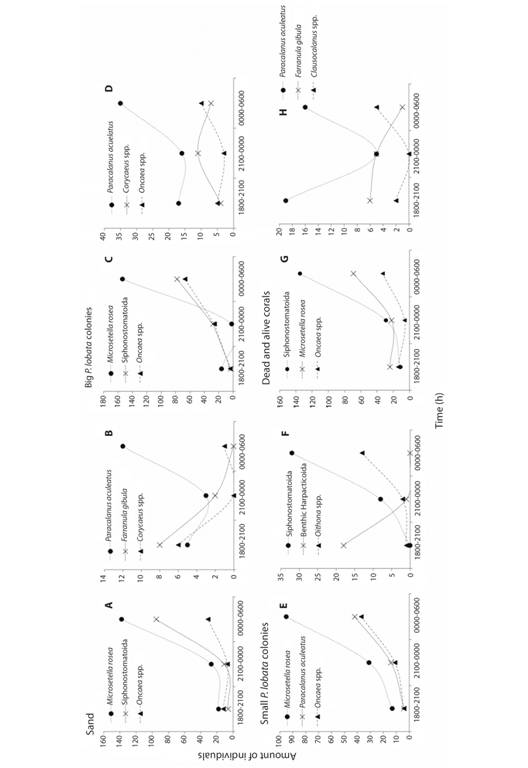
Fig. 4 Variation of the abundance of the most common copepod species during time intervals in different substrates: sand (A-B), big P. lobata colonies (C-D), small P. lobata colonies (E-F), and dead and other alive corals (G-H).
The SIMPER analysis revealed which taxa made the higher contribution for differences in community structure between different time intervals. Siphonostomatoida Order was more abundant from 0000 to 0600 h and contributed with 5% of the dissimilarities, Pterotracheoidea also contributed 5% and were only present from 0000 to 0600 h. Other important groups, each contributing with 4% to the total difference, were the decapod larvae, recorded from 1800 to 0000 h; M. rosea, found at any time but with higher abundances from 0000 to 0600 h, and amphipods, relatively constant between intervals.
Among other taxa, copepods of the family Clausidiidae, Cyclopoidae, Miracidae, the species Lucicutia flavicornis,Oithona plumifera, and also fish larvae, megalopas, Mysis shrimps, and copepodites, were only present between 0000 to 0600 h. Luciferidae, benthic copepods like Aculeopsis spp., Alteuthella spp., and Monstrillopsis chathamensis were found from 1800 to 2100 h. Others, like Undinula vulgaris and Parathalestris incerta, were only present from 2100 to 0000 h.
The sampled zooplankton communities did not show differences among the substrates (ANOSIM, R=8.60%, p=0.719). Although, the major amount of organisms were captured over big and small P. lobata colonies. Also, it was observed that some organisms were captured in just one kind of substrate, i.e. harpacticoids of the families Ameiridae and Miracidae, cyclopoids of the family Clausidiidae, the species Lucicutia flavicornis, Eudactylopus lucayosi and Oithona plumifera, and fish larvae, were found above sand. Others, as copepods of the Cyclopinidae family, the species Parapeltidium sp., Porcellidium cf. yoroium,decapod larvae (Porcellanidae), Pterotracheiodea, Phyllosoma larvae and Ostracoda, were found above the big colonies of P. lobata. On the other hand, Parathalestris cf. incerta, Dactylopodopsis cf. dilatata, Tisbe sp., Harpacticus cf. obscurus, Hyphalion sp., Idyellopsis cf. typica, and Monstrillopsis chathamensis, were foundabove small colonies of P. lobata. The copepods Mucropedia sp., Aculeopsis cf. longisetosa, Alteuthella sp., Dactylopusia sp. 1, Dactylopusia sp. 2, Eudactylopus cf.krusadensis anddecapod larvae of Luciferidae familywere found just in dead and alive coral.
Discussion
This study presents the nocturnal variation of zooplankton community structure over different substrates in a coral reef system in Chatham Bay at Isla Coco. The composition structure of demersal and pelagic zooplankton found in this study, constituted mainly by copepods, appendicularians, isopods, amphipods, polychaetes, mysids, shrimps and larval forms, coincides with the taxa stated by Porter & Porter (1977) as the one that lives in the crevices of the reef by day and migrate into the water column at night.
In this study were registered 20 species of benthic copepods which are new records, increasing from 29 to 49 species the list of benthic copepods already reported for Costa Rican waters (Morales-Ramírez, Suaréz-Morales, Corrales-Ugalde, & Esquivel-Garrote, 2014). Taking into account the role of islands as centers of species distribution and as places with a higher level of endemism (Briggs, 1966; Kier et al., 2009), it is to be expected that in Isla de Coco new records and new species can still be found. During the last decade, four new species of pelagic copepods have been recorded for Isla del Coco (Suárez-Morales & Morales-Ramírez, 2009; Suárez-Morales &Gasca, 2012; Esquivel-Garrote, Suárez-Morales, & Morales-Ramírez, 2015). There is little information about benthic copepods of Isla del Coco (Morales-Ramírez, et al. 2014), despite the existence of several studies about the benthic fauna: sipunculids and echiurans had been reported (Dean, Sibaja-Cordero, Cortés, Vargas, & Kawauchi, 2010), benthic polychaeta (Dean, Sibaja-Cordero, & Cortés, 2012), one amphioxus (Sibaja-Cordero, Trocoso & Cortés, 2012). Also, Sibaja-Cordero et al. (2016) reported some families of benthic copepods from the sandy bottoms of the island.
The most abundant organisms in this study were copepods, including nauplii, copepodites and adults. Similar results were found by Nakajima, Yoshida, Othman, and Toda (2008) in a coral reef in Redang Island, Malaysia. In this study they found copepod nauplii as the most abundant taxa and responsible for the diel migration pattern observed, where the peak of abundance was from 1800 to 2100 h, as was recorded in this study. Other studies had registered similar findings in other coral reefs, like in Tioman island, Malaysia and Conch Reef, Florida, where nauplii and copepods got the highest abundances and at night (Nakajima et al., 2009; Heidelberg et al.,2010). Higher abundances of copepods (adults, nauplii and copepodites) at night could be related to diel migration due to day time zooplanktivory by fish, which might be one of the major factors determining the diel migration pattern in coral reef zooplankton (Hamner, Jones, & Carleton, 1988).
The community structure of demersal zooplankton was more influenced by time than by the kind of substrate. In the Red Sea, differences in community composition were found while collecting zooplankton at day and night over different substrates, the differences observed were related with the time and not with the substrate (Yahel et al., 2005b). Jacoby and Greenwood (1988) also registered those differences in the planktic-benthic composition after observed migration peaks at different hours, either day or night, of different taxa like copepods (nauplii, copepodites and adult), chaetognaths, appendicularians, tanaidaceans, decapod larvae, and others. On the other hand, Olhorst (1982) stated that migration does not occur in just one pulse, and takes place at different times along the night time. However, several authors coincided that the major migration pulse happens at dusk after 1800 h (Alldredge & King, 1977; Olhorst, 1982; Yahel et al., 2005b).
Although we did not find differences among substrates, some organisms were found associated with one kind of particular substrate. According to Alldredge and Kind (1977), some organisms can keep certain positions in specific areas of the reef. Several strategies might explain this behavior: A) through active swimming; B) using crevices, caves, and coral heads as protection from predators and currents, and C) residing close to the bottom (Emery, 1968). These no significant differences between substrates could be explained because during migration, once emerged to the water column, zooplankton can be dispersed and quickly colonize a new substrate (Jacoby & Greenwood, 1988). The higher abundance of individuals found over corals could be explained by the fact that those formations give protection against physic forces and might increase the availability of food, like mucus or bacteria, which could improve the survival of demersal zooplankton on this habitat (Jacoby& Greenwood, 1988).
In conclusion, the composition and abundance of coral reef zooplankton in Isla del Coco is influenced by the diel migration pattern and not by the substrate, and this composition is similar to other coral reef systems around de world. The identification of new records or new species emphasizes the importance of Isla del Coco as a hot spot of marine biodiversity. However, it is necessary to increase the sampling effort, the time intervals throughout the day, and the use of different types of traps to establish which are the patterns of abundance, biomass, and species composition. Also, it is necessary an extra work in benthic copepod fauna sampling and identification, a group that remains poorly known both in Isla del Coco waters and along the coast of continental Costa Rica.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio