Introduction
Research on marine biodiversity lags behind that of terrestrial biodiversity in many tropical countries. The North Pacific region of Costa Rica is home to a wide variety of coastal ecosystems, including estuaries, mangroves, intertidal zones, beaches of different composition, coral reef patches and islands, which makes the area especially complex (Cortés, 2017). Within the Costa Rican North Pacific, several studies have been conducted on coral reef health and community structure, with some of them reporting high species richness in invertebrates and fishes (Dominici-Arosemena, Brugnoli-Olivera,; Espinoza & Salas, 2005; Sibaja-Cordero, Camacho-García, & Vargas-Castillo, 2014; Alvarado et al., 2018;). The area however, has faced significant urban development in recent years, and is one of the fastest growing areas for the tourist and fishing sectors in the country, contributing to the degradation of marine ecosystems and affecting their biodiversity (Morales-Ramírez, 2013; Villalobos-Rojas, Herrera-Correal, Garita-Alvarado, Clarke, & Beita-Jiménez, 2014).
The Gulf of Papagayo contains a Marine Protected Area (MPA) that is part of Santa Rosa National Park, but most of the Gulf is unprotected. Thus, fisheries operating outside the Santa Rosa MPA could affect fish and invertebrate communities. MPAs contribute to conservation and/or restoration of key species and habitats by reducing human pressure, becoming reference points for the conservation of marine communities (Strain et al., 2019). Protected areas benefit fish populations of all trophic groups, registering high biomasses of all, which can extend to nearby exploited populations (Soler et al., 2015). MPAs can also positively affect fish populations in adjacent areas through natural dispersal of juveniles (Marshall, Gaines, Warner, Barneche, & Bode, 2019).
Cabuyal and Zapotillal are two unprotected bays in the Gulf of Papagayo where fish and marine invertebrate communities have not previously been assessed. This area is of interest because of its closeness to the MPA of Santa Rosa National Park and because fisheries operate nearby. Thus, the study area could benefit from its location next to a National Park. Our aim was to evaluate the conservation status of fish and invertebrate communities of Cabuyal and Zapotillal Bays in rocky reefs and sandy areas. We assessed species richness and fish biomass by trophic group, compared densities of juvenile and adult fish and estimated species richness and density of invertebrates. Our work aims also to fill some gaps in knowledge to ultimately contribute to conserve marine life in North Pacific Costa Rica.
Materials and methods
Study site: The study was conducted during the dry season, between December 2017 and April 2018 in the bays of Cabuyal (10°67’42’’ N & 85°65’43 W) and Zapotillal (10°65’77’’ N & 86°67’13’’ W), at the southern end of the Papagayo Gulf, Northwest Costa Rica (Fig. 1). Cabuyal Bay has an area of ~ 5 km2 with reefs composed of large boulders and Zapotillal is a closed bay of ~ 530 m2 with rock platform reefs androck walls. The area is a high-energy zone with sandy beaches, and its northern and southern extremes are composed of rocky areas with cliffs. At the southern end of Cabuyal there is an estuary, associated with mangroves that receives freshwater during the rainy season from the seasonal stream Cacao (Windevoxhel Lora & Cordoba Muñoz, 1998). Zapotillal is also surrounded by well-preserved mangroves (Yaney-Keller, Santidrián Tomillo, Marshall, & Paladino, 2019), but with less freshwater inflow. Both bays are influenced by seasonal wind-driven coastal upwelling, occurring between December and April, that brings cold and productive waters to the coast (Alfaro & Cortés, 2012).
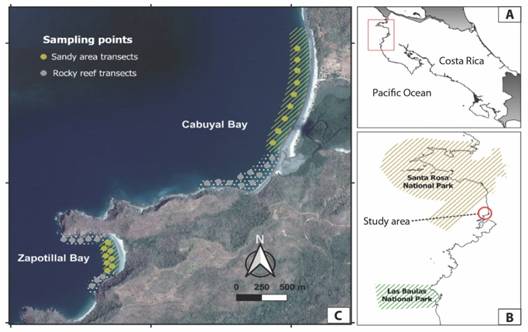
Fig. 1 Location of sampling transects in Cabuyal and Zapotillal Bays, Papagayo Gulf. A. Map of Costa Rica showing the study area. B. Location of study area in relation to marine protected areas. C. Transects carried out in sandy zones (yellow) and rocky reefs (gray) at Cabuyal and Zapotillal Bays. Dotted areas correspond to rocky reef areas sampled and lines to sandy areas. Map data property of Google Maps (https://www.google.com/maps/) and Atlas Costa Rica 2014 TEC repository (https://repositoriotec.tec.ac.cr).
Quality estimators and species richness: The inventory quality (QI), was estimated using the Clench parametric estimator model. QI represents the percentage of species that were recorded out of the total that can inhabit the area. Number of transects was used as unit effort, and data were adjusted following the Clench equation (Jiménez-Valverde & Hortal, 2003). The model was adjusted to the Simplex and Quasi Newton method to obtain parameters i and c, which allowed calculation of the slope at the end of the curve to better evaluate inventory quality (Jiménez-Valverde & Hortal, 2003). We used the following equation to determine the inventory quality:
QI = Sobs /(i/c)
Where i= intercept, c= curve slope, QI= Inventory quality and Sobs= observed species per site.
To estimate sampling effort needed to record 95 % of the fauna (n0.95) we used:
n0.95 = 0.95/[c*(1-0.95)]
We estimated species richness of the study site using four nonparametric biodiversity estimators: Jack 1, ICE and Chao 1 which consider rare species, and Bootstrap estimator. In our study we found most of the species in few sampling units, which means that if we continued sampling the species found would be rare species. Thus, these indexes give a good approximation of richness, because they take in consideration rare species that may be in the study area. Study site diversity was calculated using the Shannon index (H’); this index typically ranges between 2 and 3, with values higher than 3 representing high diversity and lower than 2 low diversity (Hill, 1973). Beta diversity was calculated with the Whittaker index (w), with values close to 1 indicating high turnover of species between two sites and values close to 0 indicating high heterogeneity of species (Whittaker, 1960).
Data collection: For fish sampling, we conducted underwater visual censuses with snorkel equipment. Eight to ten belt transects (50 m long, 5 m wide) (Fig. 1) were performed between 3 to 10 m depth (nine and ten for Cabuyal and eight and eight for Zapotillal in sandy areas and rocky reef respectively). Transects were defined uniformly throughout the study area. Two runs were made along each transect. The first run was done while the line was being unrolled, recording all conspicuous species in the water column (except the cryptic and less conspicuous ones, e.g. gobies and blennies). In the second one, the observer recorded cryptic looking species in the benthos, under rocks and between cracks. This methodology allowed us to first identify species susceptible to being frightened (Aburto-Oropeza & Balart, 2001). We estimated the size of each fish by using a PVC tube marked with intervals every five cm. Those sizes were transformed to biomass and expressed in tons per hectare (t ha-1) using the equation from Friedlander et al. (2012). To estimate biomass we used the Bayesian weight-length ratio (LWR) parameters a and b from Fishbase, based on many studies done on body shape and size (Froese & Pauly, 2020).
According to Fishbase food items, each species was classified in four different functional trophic groups: herbivores (primary consumers and detritivores); planktivorous (secondary consumers); carnivores (benthic invertebrate carnivores) and piscivores (fish predators). Fishes were classified as adult or juveniles based on maturity studies included in Fishbase, which considered length at first maturity (Lm) for different species and it range. For those species without Lm information, we used Fishbase common length size (frequent sizes for the species) to classify them. Cryptic species like blennies and gobies were all classified as adults because their sizes were in the range of maximum sizes for the species, according to Fishbase.
For invertebrates, we made 50 m band transects (50 m long, 1 m wide) to the right side of the line (ten and eight transects for Cabuyal and Zapotillal Bays respectively). Transects were done in rocky areas only, since settlements of macroinvertebrates on sandy substrates are rare. Invertebrates were classified into two groups: (1) colonies or (2) solitary invertebrates.
Colonial organisms (stony corals, soft corals, anemones, hydrozoans) and sponges were treated as circular organisms. By this way, we obtained a system of not superimposed circles on the plane to easily calculate the area covered by each one (Ulate et al., 2016). During each transect, the diver identified solitary invertebrates (mobile and sessile) that were greater than 2.5 cm. Density was estimated and expressed as organisms per square meter (org m-2). Soft corals, gorgonians, anemones, sponges, and hydrozoans were also included in density analysis. We followed Keen (1972) and Camacho-García, Gosliner and Valdés (2005) to identify mollusks, Hickman (1998) for echinoderms and Brusca (1974) and Fischer et al. (1995) for other invertebrates. Rocks or other habitat structures were not removed during the survey.
Statistical analyses: We estimated the four non-parametric biodiversity estimators using 100 sample randomizations, to eliminate the effect of order in which samples were added. To determine if there were statistical differences between densities of juvenile and adult fish, a generalized linear model (GLM) with gamma error distribution family was used, testing assumptions of normality and homoscedasticity. We used an ANOVA to compare trophic groups by bays and habitats (assumptions of normality and homoscedasticity were met). All analyses were performed using R Core Team version 3.5.2 (2018) except the non-parametric biodiversity estimators, which were inferred using the program EstimateS version 9.1.0. and Statistica version 10.0 (StatSoft-Inc., 2011). An α = 0.05 was accepted for all tests performed.
Results
A total of 27 679 specimens in 174 species were identified during the sampling period (see supplementary materials for a list of species, Digital appendix 1, Digital Appendix 2). We identified 136 species (85 fish and 51 invertebrate species) and 127 species (73 fish and 54 invertebrate species) at Cabuyal and Zapotillal Bays respectively (Table 1). Outside transects, we also identified nurse sharks (Ginglymostoma unami) but these were not included in the analysis.
TABLE 1 Number of observed species, diversity estimated with non-parametric methods (Jack 1, ICE, Chao 1, and Bootstrap), inventory representativeness by Clench model (inventory quality and n0.95) and Shannon and Beta diversity indexes for the study areas
| Faunistic indexes | Invertebrates | Fishes | |||||
| Sandy areas | Rocky reefs | ||||||
| CAB | ZAP | CAB | ZAP | CAB | ZAP | ||
| Number of observed species | 51 | 54 | 29 | 23 | 69 | 60 | |
| Jack 1 | 67 | 65 | 44 | 36 | 92 | 69 | |
| ICE | 74 | 63 | 65 | 64 | 92 | 65 | |
| Chao 1 | 63 | 56 | 41 | 27 | 84 | 69 | |
| Bootstrap | 57 | 60 | 35 | 29 | 79 | 65 | |
| Inventory quality (%) | 71 | 74 | 59 | 48 | 79 | 86 | |
| n0.95 | 83 | 51 | 124 | 171 | 54 | 25 | |
| Shannon index | 2.25 | 2.50 | 1.47 | 1.52 | 3.16 | 2.61 | |
| Beta diversity | 0.47 | 0.50 | 0.26 | ||||
*CAB and ZAP correspond to Cabuyal and Zapotillal Bays, respectively, n095: sampling effort needed to record 95 % of the fauna. Beta diversity was calculated for the exchange of species between bays.
Quality estimators and species inventory richness: Adjusting the Clench parametric model resulted in an inventory quality greater than 71 % for both fish and invertebrates in the rocky reef. However, the inventory representativeness of fish in sandy areas was less than 59 % (Table 1). Beta diversity in all the cases was below 0.50, showing differences in species composition between bays.
Fish density and biomass: Mean rocky reef fish biomass (± standard error; samples) in the study area was 1.57 t ha-1 (± 0.67 SE; N = 13 621), with 2.42 t ha-1 (± 0.04 SE; N = 4 322) in Cabuyal Bay and 0.72 t ha-1 (± 0.01 SE; N = 9 299) in Zapotillal Bay. On average, we found higher biomass in sandy areas than in rocky reefs, with 4.64 t ha-1 (± 0.19 SE; N = 6 038) and 3.04 t ha-1 (± 0.29 SE; N = 3 466) for Cabuyal and Zapotillal sandy areas, respectively. There were statistically significant differences between biomass of trophic groups (F = 14.7, d.f. = 583, P < 0.001). Biomass of piscivorous fishes was higher in rocky reefs, followed by carnivorous fishes, in both Cabuyal and Zapotillal Bays (Fig. 2A). Sandy areas had a higher biomass of piscivorous and planktivorous fish (Fig. 2B). A total of 13 931 fishes were classified as adults, while 9 194 were juveniles including both sites. Mean densities of adults were higher than that of juveniles at both study sites (Fig. 2C). These differences were statistically significant at Cabuyal Bay (X2= 5.04, d.f. = 1, P < 0.05), but not at Zapotillal Bay (X2 = 0.01, d.f. = 1, P = 0.91).
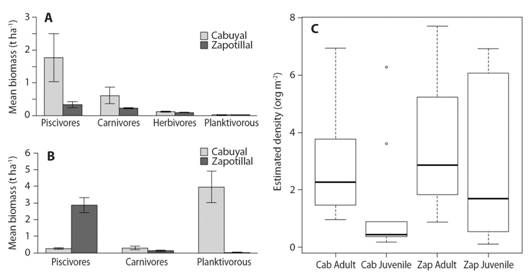
Fig. 2 Mean estimated biomass of trophic levels with standard error bars in A. the rocky reefs and B. sandy areas, and C. Mean density of developmental stages adults and juveniles in Cabuyal (Cab) and Zapotillal (Zap) Bays (box represents 1st quartile (top of box) and 3rd quartile (bottom of box) with the black line indicating median value. Whiskers represent minimum (lower) and maximum (upper) with outliers shown as open circles).
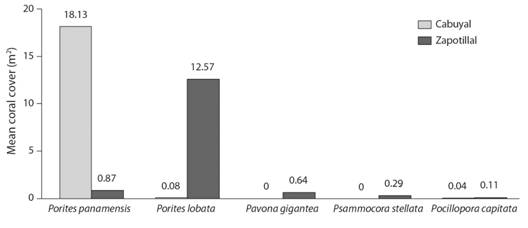
Coverage of colonial organisms and density of invertebrates: we identified a total of 11 coral species dispersed on the rocky reef, of which 7 species were found in Zapotillal Bay (5 hexacorals and 2 octocorals) and 6 species in Cabuyal Bay (3 hexacorals and 3 octocorals). One hexacoral of the order Scleractinia could not be identified in Cabuyal Bay during the sampling. Mean coral cover in the substrate for the study area was 4.09 % (± 2.51 SE; N = 50), with a mean of 11 % (± 0.21 SE; N = 21) in Cabuyal Bay and 4 % (± 0.09 SE; N = 29) in Zapotillal Bay. Porites panamensis and Porites lobata represented the greatest mean coral cover in the study area (Fig. 3). Other colonial organisms such as soft corals, gorgonians, sponges, hydrozoans, and anemones comprised a mean cover of 5 % (± 0.40 SE; N = 230) and 4 % (± 0.32 SE; N = 206) for Cabuyal and Zapotillal Bays, respectively.
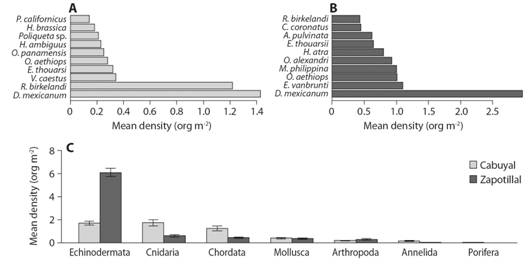
Echinoderms were the group of invertebrates with the highest mean density followed by chordate, mollusks, and arthropods (Fig. 4A, Fig. 4B). At the species level, the highest mean density was sea urchin Diadema mexicanum and ascidia Ropalaea birkelendi in Cabuyal Bay, and sea urchin D. mexicanum and Echinometra vanbrunti in Zapotillal (Fig. 4A, Fig. 4B). In general, the dominant mobile invertebrates in Zapotillal had a higher density of organisms per square meter than those dominant in Cabuyal. Among solitary invertebrates, seven species could not be identified.
Discussion
Quality estimators and species richness: The quality ofour inventory was high in rocky reefs as proportions above 70 %, indicate that estimates of asymptotic richness are stable (Jiménez-Valverde & Hortal, 2003). However, ~ 50 % of fish species may not have been recorded in sandy areas. Due to their very dynamic nature, many species move through sandy zones with the tide (Herrera & Bone, 2015; Andrade-Vera, Domínguez-Granda, & Marín-Jarrín, 2017), likely reducing inventory representativeness.
Espinoza and Salas (2005) and Dominici-Arosemena, Brugnoli-Olivera, Cortés-Núñez, Molina-Ureña and Quesada-Alpizar (2005) respectively reported 46 and 75 fish species in rocky reefs in the Papagayo Gulf. We found a higher number of species (81) following a different methodology. Our methodology included doing a second round to account for cryptic fish species, which likely resulted in a more complete inventory. At rocky reefs, we identified more invertebrates (70 species) than previously reported for coral reefs (38 species) in North Pacific Costa Rica. Our results suggest that rocky reefs in North Pacific Costa Rica hold a high number of species, however reefs have been strongly affected by human impacts in the region in recent years (Alvarado et al., 2018). MPAs are not always located in the areas with the highest biodiversity or are not large enough to fully protect highly diverse areas, leaving unprotected areas of high biodiversity subject to human exploitation (Sibaja-Cordero et al., 2014), as could be the case of the study sites.
Beta diversity showed that communities of both bays had different species compositions of both fish and invertebrates. Rocky areas are complex ecosystems with high heterogeneity in composition and structure, offering shelter to a wide number of species (Dominici-Arosemena & Wolff, 2006; García-Hernández et al., 2014). Boulders and walls such as those present in the study area, are characterized by a high number of crevices and rugosities favoring the settlement and refuge of different species (Aburto-Oropeza & Balart, 2001). Furthermore, environmental factors such as the input of sediments and nutrients from mangroves can also shape species composition, favoring organisms like the ascidian R. birkelandi found in Cabuyal Bay (Dittmar & Lara, 2001). The biological diversity of the substrate also favors settlement of other species (Aburto-Oropeza & Balart, 2001). Coral coverages and organisms such as sponges, anemones, hydrozoans, and soft corals like those found in the study area, provide substrate for recruitment, feeding habits and refuge from predation (Ferreira, Goncçalves, & Coutinho, 2001).
Biomass, trophic groups, and juvenile fishes: reef fish biomass at Cocos Island National Park (Costa Rica), a biodiversity hotspot highly protected from fishing, was estimated as 12.5 t ha-1 (Fourriére et al. 2019), much higher than what we found (1.57 t ha-1). However, North Pacific Costa Rica has been subjected to habitat loss and intense fishing and fish biomass here is much lower (1.5 t ha-1Alvarado et al., 2018) and similar to our findings. This suggest that Cabuyal and Zapotillal Bays, similarly to other areas in the region, (Villalobos-Rojas et al., 2014), have suffered impacts from fishing and habitat loss.
MPAs benefit all trophic groups, but recovery is usually faster in large carnivorous fish due to their rapid growth (Soler et al., 2015). Protection results in a high biomass of mobile fish that can also affect surrounding areas (Beita-Jiménez et al., 2019). We found higher biomasses of piscivorous and carnivorous fish in rocky reefs, which could suggest a possible impact from the nearby MPA of Santa Rosa. However, except for piscivorous fishes in Cabuyal Bay, we found lower biomass of fish in all trophic groups than those reported for other unprotected areas in the region (Beita-Jiménez et al., 2019). Despite being close to a MPA, fish populations of Cabuyal and Zapotillal Bays seem affected by fisheries, possibly as a result of lack of regulations and low level of compliance in the region (Arias, Cinner, Jones, & Pressey, 2015).
While predatory fishes are most heavily exploited, herbivores are also targeted by artisanal fisheries, which may lead to reduced grazing of macroalgae, negatively affecting live coral cover (Edwards et al., 2014). In Costa Rican North Pacific, coral cover has declined from 43 to 5 % in the last 15 years, and reefs have become macroalgae dominated (Alvarado et al., 2018). This leads to high densities of other herbivores such as the sea urchin D. mexicanum, which affects coral recruitment (Alvarado, Cortés, & Reyes-Bonilla, 2012). Thus, the low coral cover and high density of D. mexicanum that we found could be explained by a low biomass of herbivorous fish, similar to that reported by Alvarado et al. (2018).
We found high densities of juvenile fishes, especially at Zapotillal Bay. Alvarado et al. (2018) also reported a high percentage of juveniles in several reefs of North Pacific Costa Rica. MPAs are known to benefit fish recruitment, exporting juveniles to surrounding areas (Marshall et al., 2019). In addition, North Pacific Costa Rica is rich in rocky ecosystems (Cortés, 2017), which provide a variety of shelters to avoid pelagic and benthic predators (Moreno Pérez, 2019). Thus, Zapotillal Bay seems important for recruitment and breeding of fish populations, especially considering that the area is impacted by fisheries. Our study reports for the first-time fish biomass for sandy areas in the Papagayo Gulf. The presence of big schools of piscivorous and planktivorous fishes with high biomasses characterized the site. Sandy areas are considered heterogeneous and complex habitats (Moreno Pérez, 2019), so further studies are needed to describe species composition, as well as their dynamics.
Implications for conservation: This is one of few studies done on fish and invertebrate communities of rocky reefs and sandy areas in the Papagayo Gulf. We found high biodiversity of fish and marine invertebrates in rocky reef environments and identified some invertebrates threatened by overfishing such as the green lobster Panulirus gracilis and the sea cucumber Isostichopus fuscus ( Naranjo-Madrigal, 2011; Mercier, Toral-Granda, Alvarado, & Benavides-Serrato, 2013). The area could be benefitting from its closeness to the Santa Rosa MPAs. Densities of juvenile fish were especially high in Zapotillal Bay, suggesting that the area may be a breeding site contributing to the maintenance and resilience of reef fish populations. On the other hand, the area seems to be under fishing pressure, affecting all trophic groups. The low coral cover found also confirms that coral ecosystems are highly affected by degradation along the North Pacific coast of the country, mainly by eutrophication, overfishing and urban development (Alvarado et al., 2018). Our findings contribute to the description of marine biodiversity in rocky reef and sandy areas of the Papagayo Gulf. We expect that this information will serve for initiatives such as the BioMar Costa Rica project to increase knowledge of the country’s marine biodiversity, which will ultimately contribute to the conservation of marine life in the region.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio