Introduction
Land molluscs are vulnerable to dehydration because their skin is highly permeable and because they move by laying down a band of moist mucus (Hyman, 1967; Prior, 1985; Moreno-Rueda, Ruiz-Ruiz, Collantes-Martín, & Arrébola, 2009). Therefore, seasonality, activityand microhabitat selection are important for their survival (Prior, 1985; Cook, 2001).
Regarding seasonality, in temperate regions, many terrestrial gastropods exhibit a decline in abundance in summer and winter, sometimes because they become inactive for months and others because they die (Hyman, 1967; Cook, 2001; Reyes-Tur, Flores-Ricardo, & Fernández-Velázquez, 2018). In tropical areas with well-defined rainy and dry seasons, land snail abundance also decreases during the driest months (Barrientos, 2000; Suárez & Fernández, 2012). Activity in most land snail species, including tropical species, has been reported as nocturnal, but there are some reports of diurnal species (Cook, 2001; Barrientos, 2016; Reyes-Tur et al., 2018).
Few aspects of microhabitat selection have been studied. In arid Mediterranean regions the choice is regulated by humidity and temperature, but in humid places only temperature is meaningful (Moreno-Rueda et al., 2009). However, other researchers state that litter moisture and litter level, shading, ground water level and abundance of some plants are also important in microhabitat selection (Willig, Sandlin, & Gannon, 1998;Barrientos 2000; Książkiewicz, Kiaszewicz, & Gotdyn, 2013; Barrientos 2019b). Regarding microhabitat selection of tropical arboreal snails, Hernández-Quinta (2013) found that most Cuban species prefer branches and trunks, although some species can be found on leaves, except for Jeanneretia bicinta which is restricted to leaves. However, there are no studies that explain why, when and in which part of the leaf J. bicinta prefers to be.
In tropical environments, seasons are defined by rainfall rather than temperature variations. The continental rainfall regime has its main peak between 15:00 and 18:00 h (Yang & Smith, 2006; Kikuchi & Wang, 2008). In tropical montane wet forest, it rains even during dry seasons and leaflitter moisture is high and constant around the year, creating a humid environment inside the forest (Barrientos, 2012). Under these circumstances, at night, temperature decrease helps reaching dew point and water drops will form and gather in the canopy surface, producing canopy drip (Herwitz, 1985; Germer, Elsenbeer, & Moraes, 2006). Throughfall can also cause canopy drip, this is the reason why drops fall inside the forest, even though rain has stopped. Most raindrops have a diameter of 1 to 3 mm, but they may reach four millimetres (Levia, Hudson, Llorens, & Nanko, 2017). Canopy drip drops are bigger reaching a diameter of 7 mm depending on meterological conditions and, canopy and leaf density and geometrics (Levia et al., 2017). Rainfall, throughfall, dew and canopy drip may affect directly the amount of fresh materials eaten by snails, and indirectly snail quantity, aestivation and activity patterns, among other (Barrientos, 2000; Wiesenborn, 2003; Mensink & Henry, 2011; Książkiewicz-Parulska & Ablett, 2016). Beside this, drop size and kinetic energy may be important threats to small species, however, they have not been considered in tropical land snail behavior analyses (Herwitz, 1985; Germer et al., 2006). Maybe the earliest author to mention the negative effect of raindrops, dew and canopy drip was the Roman naturalist Pliny the Elder in 74 CE (Plinius, 1855).
The genus Tikoconus has seven described species; all of them endemic to Costa Rica. They live in very humid forests from 400 m.a.s.l. on the Caribbean slope -and 760 m.a.s.l. on the Pacific slope- to 2 500 m.a.s.l. (Barrientos, 2019a). The land snail T. costarricanus is a 12 mm long semislug with a 4.6 mm diameter shell and dwells on understory shrubs in tropical montane wet forest (Barrientos, 2019a). In this paper, I analyze its season-dependent daily activities, leaf side selection and the importance of rainfall and canopy drip in their daily behavior.
Materials and methods
I conducted this study in a mature forest, or “old growth forest” according to Clark (1996), near “El Llano” water dam (9º45’56.07” N & 83º51’47.11” W, 1 640 m.a.s.l.), in a tropical lower montane wet forest, Reserva Forestal Río Macho (Costa Rica). I observed daily activity of T. costarricanus specimens on a 2 km long trail during seven 24 h periods (25 May 2010, 7 September 2010, 20 October 2010, 30 April 2010, 31 January 2011, 21 February 2011, 21 March 2011). I observed 167 specimens and made 781 observations in total. During the first sampling of each day I walked along the trail searching for snails and marked the plant and leaves with red ribbon, and the snail´s location on the leaf, with a white ink marker. This allowed me to find them easily in the next samplings and record their displacement. I sampled every 3-4 h in a 24 h period, each sampling lasted 1 to 2 h.
All specimens considered were adults or sub adults with a well pigmented cephalopodium and a shell diameter of 3.5 mm or more (Barrientos, 2019b). Whenever I found an individual I observed the specimen for less than 15 s, disturbing it as little as possible and took note of: season, time, activity, behaviour, temperature and relative moisture inside the forest (only during dry season samplings due to logistic problems), part of the leaf, plant height (five categories: 0-0.5 m, 0.6-1.0 m, 1.1-1.5 m, 1.6-2.0 m, 2.1-2.5 m) and weather. I divided weather in three categories: rainy (rain and drizzle), cloudy (cloudy with wet soil) and sunny (cloudy with dry soil or sunny) (94 % of all sunny records took place before 13:00 pm). I classified time in eight categories: 05:00 am - 07:59 am, 08:00 am - 10:59 am, 11:00 am - 13:59 pm, 14:00 pm - 16:59 pm, 17:00 pm - 19:59 pm, 20:00 pm - 22:59 pm, 23:00 pm - 01:59 am and 02:00 am - 04:59 am. I considered that specimens were active when optical tentacles were extended. Active specimens were “still” when optical tentacles were extended but the snail was not moving (Fig. 1A). Other behaviours that they could be doing while active were: eating, defecating, moving or grooming (Barrientos 2020). I considered specimens “inactive” when optical tentacles were withdrawn in the body. Inactive specimens could be: “resting”, when tentacles were withdrawn, cephalopodium was extended and the whole foot sole was in contact with leaf surface; or “aestivating in bat-like hanging position” (for details about this position see Barrientos 2020) (Fig. 1B).

Fig. 1 A. Active still snail; this specimen was not moving but its optical tentacles were extended. B. Inactive resting snail; this specimen´s optical tentacles were withdrawn and the whole foot sole was in contact with the surface.
I analysed the daily rainfall pattern in data obtained by the Instituto Costarricense de Electricidad (ICE) meteorological stations number 73029 El Llano (9°45’52.87788” N & 83°51’29.99196” W, 1 583 m.a.s.l.), located 1.4 km East from the sampling place. In the analysis I included data from January 1, 2010 to January 2, 2012. I considered data from December 1 to April 30 as dry season and from May 1 to November 30 as rainy season. I calculated the rain average per h for the dry and the rainy season with data from El Llano station.
Voucher specimens: I deposited voucher specimens in the Zoology Museum of the University of Costa Rica. Catalogue number MZUCR- 242-01 (9 specimens). Costa Rica, Cartago, Orosi, Reserva Forestal Río Macho, at “El Llano” water dam (9º45’56.07” N & 83º51’47.11” W, 1 640 m.a.s.l.) collected on 5 March 2012 by Zaidett Barrientos, Maribel Zúñiga & Andrea Induni (T.: ZB-251).
Results
Substrate preference and activity: Regardless of the season most specimens can be found on leaves (98.5 %, sample size = 781), the rest were on twigs. Therefore, for the rest of the analysis I considered only specimens that were on the upper side and on the underside of leaves (sample size = 769). Only 24 % of the snails were on the upper side of leaves (X2 = 203.92, d.f. = 1, P < 0.0001, sample size = 769); most were active (92 %) (X2 = 129.16, d.f. = 1, P < 0.0001, sample size = 186). In contrast, most inactive snails were on the underside of leaves (95 %) (X2 = 263.98, d.f. = 1, P < 0.0001, sample size = 323). But those that were on the underside of leaves, were active or inactive in the same proportion (X2 = 1.76, d.f. = 1, P = 0.1846, sample size = 583).
Activity according to daytime and season: For this analysis I did not consider the leaf side in which the specimens were.This species can be active at any time of the year and the day (Fig. 2A and 2C).
In the rainy season activity was high during light hours (58 to 87 %), specially between 8:00 and 10:59 am, and declined at night and wee hours (20:00 pm-4:59 am) (X2 = 30.59, d.f.= 7, P = 0.00011, sample size = 266) (Fig. 2A). Nevertheless, there was a second activity peak during the dark hours (17:00 to 19:59 pm) and even in the less active periods at least 30 % of the snails were active.
In contrast, in the dry season, activity was high between 5:00 to 10:59 am, but declined during noon and afternoons (11:00 am to 16:59 pm) (X2 = 91.53, d.f. = 7, P < 0.0001, sample size = 503) (Fig. 2C). During dark hours there was a second activity peak (17:00 to 19:59 pm). Even in the driest period (14:00 to 16:59) at least 12 % (sample size = 50) of the specimens were active (Fig. 2C). Activity during dark hours was high (54 to 82 %).
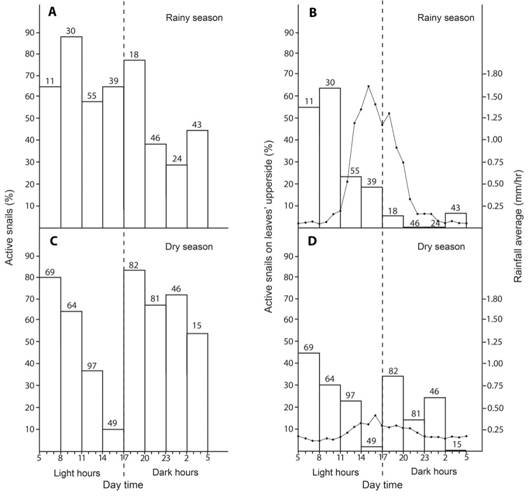
Fig. 2 Activity of T. costarricanus according to season and daytime. A. and B. Active snail percentage in any part of the leaf. C. and D. Active snails’ percentage on the upper side of leaves. Numbers above bars are the sample size for a given time category. There are no variation bars because these are total numbers.
Active snails leaf-side preference: The side of the leaf in which specimens were active varied with daytime and the season (X2 = 51.66, d.f. = 7, P < 0.0000, sample size = 769 records, 503 were from the dry season) (Fig. 2B and Fig.2D).
Rainy season: The “active snail percentage” and “active snails on the upper side of leaves” patterns were considerably different (Fig. 2A and 2B). Similar to the activity pattern during light hours I found the highest percentage of active snails on the upper side of leaves between 8:00 and 11:00 am. But in contrast, active snails on the upper side of leaves had any activity peak during dark hours (X2 = 23.36, d.f. = 7, P = 0.0015, sample size = 266) (Fig. 2B). During light hours, active snails´ percentage on the upper side of leaves had an inverse correlation with rainfall (Fig. 2B). Rainfall average in the rainy season was 0.5 mm per h (maximum = 42.4 mm/h, minimum = 0 mm/h, sample size = 10 271).
Dry season: “Active snail percentage” and “active snails on the upper side of leaves” patterns have similar shapes in the dry season (X2 = 12.9, d.f. = 7, P = 0.075, sample size = 266) (Fig. 2C and Fig.2D), although active snail percentage on the upper side of leaves was lower, especially during dark hours (Fig. 2D). Active snail percentage on the upper side of the leaves had two activity peaks similar to the active snail´s pattern (Fig. 2C and Fig.2D). Rainfall average in the dry season was 0.2 mm per h (maximum = 13 mm/h, minimum = 0 mm/h, sample size = 7 010).
In the dry season light hours, “snail activity” and “active snails’ quantity on the upper side of leaves” decreased with humidity decrease but mainly by temperature increase (Fig. 3). During dark hours, high and almost constant humidity was related with more active snails (Fig. 3A). In contrast, temperature decreased during dark hours was related with a lower percentage of active snails on the upper side of leaves (Fig. 3B). In this season, inside the forest, relative humidity had the lowest average and temperature the highest average between 10:00 am and 16:59 pm (Relative humidity: Ø = 84.65, min = 65.3, max = 96.8, S.D. = 7.5, sample size = 103) (Temperature: Ø = 17.6, min = 12.3, max = 22.7, S.D. = 1.7, sample size = 103) (Fig. 3). On the contrary the highest relative humidity and lowest temperature inside the forest took place from 17:00 pm to 9:59 am (Relative humidity: Ø = 90.6, min = 71, max = 99, S.D. = 4.2, sample size = 310) (Temperature: Ø = 15.3, min = 11.11, max = 19.3, S.D. = 1.9, sample size = 310) (Temperature: U-Mann-Whitney (Wilcoxon): W = 5226, P = 0, sample size = 403) (Relative humidity: U-Mann-Whitney (Wilcoxon): W = 23193, P < 0.0001, sample size = 403) (Fig. 3).
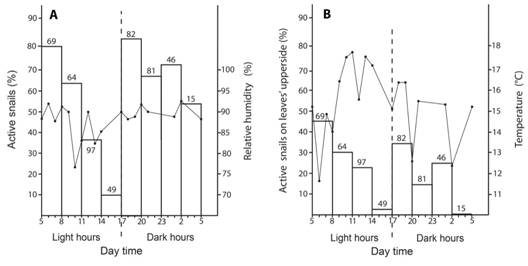
Fig. 3 A. Active T. costarricanus according to relative humidity inside the forest during dry season (relative humidity sample size = 460). B. Active T. costarricanus on the upper side of leaves according to temperature inside the forest during dry season (temperature sample size = 460). Numbers above bars are the sample size for a given time category. There are no variation bars because these are total numbers.
Activity according to weather: Patterns differed dramatically between dry and rainy seasons (X2 = 40.29, d.f. =2, P < 0.0001, sample size = 769; 503 of them in the dry season) (Fig. 4A). In the rainy season there were more active specimens with drier weather, but in the dry season there were more active snails with wetter weather (Fig. 4A).
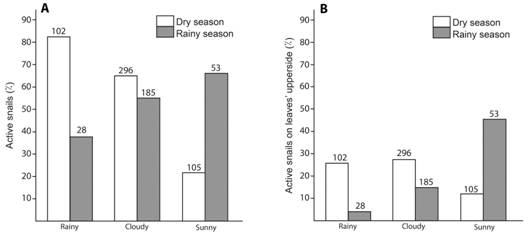
Fig. 4 Activity of T. costarricanus according to season and weather. A. Active snails without considering the place in which specimens were. B. Active snails on the upper side of leaves. Numbers above bars are sample size. There are no variation bars because these are total numbers.
Activity according to weather and side of the leaf: When I considered the side of the leaf in which active snails were, I found that it was also season-dependent (X2 = 33.73, d.f. =2, P < 0.001, sample size = 769; 503 of them in the dry season) (Fig. 4B). In the rainy season the pattern is similar to the activity pattern (Fig. 4A and Fig.4B). In the dry season I found less snails on the upper side of leaves during sunny periods, but the snail percentage of active snails on the upper side of leaves was similar in rainy and cloudy days (Fig. 4B).
Other seasonal behaviors: Regarding active snails, I found seasonal differences only for the leaf side in which active snails prefer to be “still” (X2 = 4.68, d.f. = 1, P = 0.03, sample size = 323). During the dry season 35 % of the “still” snails were on the upper side of leaves (sample size = 229), while only 17 % of them were on the upper side of leaves during the rainy season (sample size = 94).
Regarding inactive snails, I found that few specimens were on the upper side of leaves during the dry season 6 % (sample size = 205) and even fewer during the rainy season 2 % (sample size = 118). I found bat-like aestivating snails only on the underside of leaves. They were more common in the dry season (86 %, sample size = 153) (X2 = 35.61, d.f. = 1, P < 0.0001, sample size = 769). Beside this, in both seasons most snail aestivated around noon (X2 = 1.57, d.f. = 3, P < 0.6662, sample size = 769) (Fig. 5). I never found aestivating specimens between 2:00 am and 4:59 am, regardless of the season. However, during the dry season aestivating specimens had a wider distribution from 5:00 am to 1:59 am, but 92 % (sample size = 132) aestivated between 8:00 am and 16:59 pm. During the rainy season, I found all aestivating specimens between 8:48 am and 12:55 pm, especially around noon (Fig. 5). Any individual aestivated more than 21 h in a row.
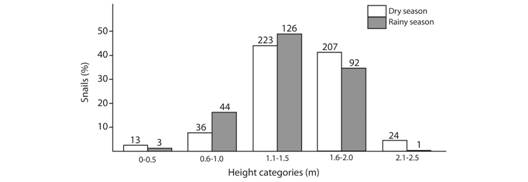
Vertical preference according to season: Vertical distribution also differed among seasons (X2 = 29.13, d.f. = 4, P < 0.0001, sample size = 769). In both cases I found more specimens between 1.1 m and 2.0 m, during the dry season there were more specimens in the lowest and highest layers when compared to the rainy season (Fig. 6).
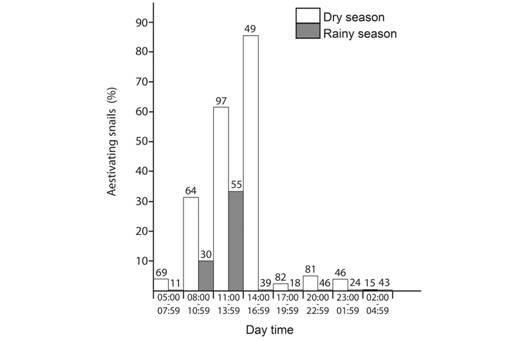
Fig. 5 Bat-like aestivating T. costarricanus according to season. Numbers above bars are sample size. There are no variation bars because these are total numbers.
Discussion
In contrast to most land snails, the neotropical snail T. costarricanus has the same abundance throughout the year, is active at any time of the day or night and spends most of time on leaves (Barrientos, 2019b). However, some of its behaviors and preferences are seasonal and also vary along the day.
Many active T. costarricanus snails seem to avoid leaves´ upper side, even though food is more abundant there and their pigmentation help them camouflage with epiphytes, suggesting that there are difficulties on this side of the leaves (Barrientos, 2019b, Barrientos, 2020). Besides this, snail frequency on the upper side of leaves changes with season and daytime. There are other land snails that show leaf side preference (Hernández-Quinta, 2013), but it is not known if preference changes with age, season or daytime. Hernández-Quinta (2013) suggested that leaf side preference may represent a strategy against adverse climatic conditions, predators, or simply a place for protected rest. In the case of T. costarricanus, probably due to their reduced size and dehydration susceptibility, specimens avoid being on the upper side of leaves due to three reasons: 1) direct sunlight impact that cause water loss and induce throwing themselves to the ground (Barrientos, 2020); 2) low relative humidity, which induce aestivation on the underside of leaves (Barrientos, 2020); and 3) rain and canopy drip, which force them to find a protected place on the underside of leaves. Why are rain and canopy drip so significant?
Rain drops diameter are about 2 mm, but during a shower, these 12 mm snails have to face many drops falling close to each other with kinetic energy during a short period (Levia et al., 2017; Barrientos, 2019a). In the case of canopy drip the kinetic energy may be less, the temporary and special distance between drops may be bigger, but drops are bigger than the snail´s shell (Levia et al., 2017; Barrientos, 2019a). Water surface tension of drops may trap and drown snails, but also drops´ kinetic energy may throw the snail from the leaves into the leaf litter. If this happens, they may: 1) fall into a flooded area and drown; 2) fall on leaf-litter that has lost their protection function against predators and weather due to rainfall compaction effect; T. costarricanus has been found in the leaflitter only during the dry season (Barrientos, 2012, Barrientos, 2019b); or, 3) have to spend extra energy to get back onto the leaves where they feed. Therefore, when water drops may fall, it is more convenient to stay in a protected place, in this case, the closest rain protected spots are leaves´ underside (Pesquero, Corrêa, Pesquero, & de Paula, 2014). Experiments with mosquitoes have shown that specimens struck by raindrops may achieve accelerations from 100 to 300 gravities. The small mass of mosquitoes allows them to survive if they are flying. But they die if: 1) they are on immobile surfaces; 2) the distance to the ground is not enough to recover from the impact of a raindrop; or 3) they fall into a puddle as they drown due to adhesion to the water surface (Dickerson, Shankles, Madhavan, & Hu, 2012).
Rainfall peak range at “El Llano” water dam is similar to nearby stations, but five hours longer than expected for a continental regime (Yang & Smith, 2006; Kikuchi & Wang, 2008; Sáenz, 2014). This means that the exposure time to raindrops and canopy drip is much longer than in other habitats. During the rainy season, rainfall peak pattern explains why T. costarricanus avoids being on the upper side of leaves during afternoons. However, rain alone cannot explain the small snail number on the upper side of leaves during night and wee hours. The pattern is better explained, if we also consider canopy drip from rain and from dew condensed on leaves’ surface. Direct raindrop impact probably explains why this snail also avoids the higher height category, while splash out on the soil explains why they also avoid the lower height category during the rainy season. Snails preference to be on leaves between 1.1 and 2.0 m could also be a consequence of leaf abundance, a possibility that has to be analyzed in future projects.
During the dry season, relative humidity, temperature, dew and fog precipitation are more important than rain to understand the leaf side preference of T. costarricanus. Although rain precipitation matches with the active snail percentage on the upper side of leaves decrease in the afternoons, the comparison with the rainy season shows that it is not the main explanation. In the dry season, relative humidity increases late in the afternoon (17:00pm) when temperature starts to descend (Gómez-Sanz, 2004; Aparecido et al., 2018; Abdalah-Hernández, Rodríguez-Yáñez, & Alvarado-González, 2020). This allows snails to forage on the upper side of leaves during late afternoon and night. At night around eight and in the wee hours, dew point is reached due to high and almost constant relative humidity and temperature drop, forming fog and dew (Aparecido et al., 2018). Fog precipitation contribution is higher during the dry season (Holder, 2004), contributing to canopy drip and preventing snails to forage on the upper side of leaves during wee hours. Early in the morning, temperature raises and the sun evaporates the moisture accumulated during wee hours stopping canopy drip from dew and fog precipitation; allowing the snail to forage again. Along the morning temperature continues to rise and the number of snails on the upper side of leaves decreases as well as the relative humidity (Abdalah-Hernández et al., 2020). During this period, lack of moisture and, maybe, direct sunlight, forced them to aestivate some hours (Barrientos, 2020). The cycle starts again around sunset when temperature falls.
The consequences of being driven off from leaves by splash out, rain, canopy drip from throughfall or dew and fog precipitation also explain why: 1) almost all snails on the upper side of leaves were active; 2) almost all inactive snails were on the underside of leaves; and 3) more resting but alert snails (“still”) were found on the upper side of leaves during the dry season.
Information in this paper shows that analyses of rainfall interaction with snail activity are strongly needed and should also consider canopy drip, dew, throughfall, fog precipitation and splash out from soil.
Ethical statement: the author declares that she agrees with this publication; that there is no conflict of interest of any kind; and that she followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio