Introduction
Entomopathogenic fungi are considered important natural pathogens of arthropods and are used mainly for the biological control of insect pests. These fungi have demonstrated a promising ability to colonize endophytically a wide variety of plant species (Wilson, 1995). This feature provides multiple benefits to plants that include double protection against insect pests and plant diseases (Jaber & Ownley. 2018), and growth promotion (Jaber & Enkerli, 2017). The presence of entomopathogenic fungi naturally occurring as endophytes also demonstrates that they have complex life cycles, which can be completed either in the soil, invertebrate organisms or plants. Besides the taxonomy and phylogeny of fungal entomopathogens, most research has focused on their development as biological control agents (Vega, 2008). Recently studies have examined their biology, including the survival and interactions outside the host insects (Mantzoukas, Chondrogiannis, & Grammatikopoulos, 2015; Manoussopoulos, Mantzoukas, Lagogiannis, Goudoudaki, & Kambouris, 2019; Mantzoukas & Lagogiannis, 2019). Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) is an entomopathogenic fungus widely distributed worldwide (Ownley et al., 2008). This fungus has been isolated as a naturally occurring endophyte from different plant species (Vega, 2008). In addition, it has been introduced artificially by using different techniques in corn (Wagner & Lewis, 2000; Russo, Pelizza, Cabello, Stenglein, & Scorsetti, 2015), soybean (Russo et al., 2018), potato (Jones, 1994), cotton (Jones, 1994), jimsonweed (Jones, 1994), tomato (Allegrucci, Velazquez, Russo, Perez, & Scorsetti, 2017), date palm (Gomez-Vidal, Lopez-Llorca, Jansson, & Salinas, 2006), banana (Akello, Dubois, Gold, Coyne, Nakavuma, & Paparu, 2007), coffee (Posada, Aime, Peterson, Rehner, & Vega, 2007) and cocoa (Posada & Vega, 2005), with the aim of evaluating its potential for the biocontrol of insect pests.
Species of the genus Capsicum are likely the most consumed peppers worldwide (Rozin & Schiller, 1980). Capsicum annuum L. is one of the five economically most profitable species in the world (Perry et al., 2007). The aphid Myzus persicae (Hemiptera; Aphididae) has a worldwide distribution and infests more than 40 plant families (Blackman & Eastop, 2000). This aphid is one of the main pests of C. annuum and has the ability to transmit more than 100 plant viruses and cause direct damages in pepper plants. In many crops, its control is based almost exclusively on the application of insecticides and, as a result, this species has developed multiple resistance to many chemical classes, including organophosphates, carbamates, and pyrethroids (Devonshire et al., 1998). Laboratory studies have shown that some strains of B. bassiana are highly virulent against M. persicae and are able to infect almost 95 % of the aphids by direct application (Chen, Li, & Feng, 2008).
The aims of this study were i) to evaluate the most effective inoculation techniques, (leaf spraying, seed immersion and root dipping) for the endophytic inoculation of B. bassiana (LPsC 1067) in pepper plants. ii) To evaluate the presence of natural endophytes in inoculated leaf and its interaction with B. bassiana. iii) To assess the effect of B. bassiana on M. persicae pepper leaf feeding preference and the growth of the aphid pest.
Materials and methods
Fungal isolate: The strain B. bassiana LPSC 1067 (GenBank accession number KF500409), isolated from Coleoptera, Coccinelidae, was obtained from the culture collection of “Instituto Spegazzini” (Universidad Nacional de La Plata), La Plata, Buenos Aires, Argentina. The selection of this fungal strain was based on its effectiveness in laboratory tests against other insect pests common in Argentina (Pelizza et al., 2012) and for its endophytic capacity previously evaluated on other crops of agronomic importance (Allegrucci et al., 2017).
Fungal suspension: Conidia were obtained from 14 day cultures grown on potato dextrose agar (PDA, Britania S.A., Buenos Aires, Argentina) at 25 °C in the dark. The conidia were harvested with disposable cell scrapers (Fisherbrand®) and placed into test tubes containing 0.01 % (v/v) Tween 80 (polyoxyethylene sorbitan monolaurate solution, Merck®). The suspensions were vortexed for 2 min and then filtered through four layers of sterile muslin. The conidial concentration was determined using a Neubauer hemocytometer and adjusted to 1×108 conidia/ml (Gurulingappa, Sword, Murdoch, & McGee, 2010). To evaluate the conidial viability before each experiment, 0.3 ml of each dilution was placed onto a slide with a thin layer of APG culture medium (Goettel & Inglis, 1997). The slides were incubated in a humid chamber at 25 °C in the dark for 24 h. Afterward, the slides were examined under the optical microscope. Conidia were considered germinated when the germ tube had at least the same diameter as the spore. This germination test was performed with three repetitions for each suspension to standardize the viability evaluations. In all cases, the suspensions used presented conidia viability greater than 95 %.
Host plants: Pepper seeds (C. annuum L) were obtained from organic crops nearby the city of La Plata, Buenos Aires province, Argentina (34°57’17” S & 57°53’26” W). None of these seeds received previous chemical treatment. To ensure the absence of fungi on their surface, seeds were surface-sterilized by placing them in 70 % ethanol for 2 min, washed in sterile distilled water, then submerged in 0.5 % sodium hypochlorite for 1 min and rinsed again in sterile distilled water (Brownbridge, Reay, Nelson, & Glare, 2012). Thereafter, the seeds were left to dry for 30 min on sterile filter paper. Half of the seeds were used for seed inoculation and the other half was planted for the application of foliar spraying and root dipping techniques after the emergence of the seedlings. The seeds were placed for germination in plastic pots with 86 g of an equally ground mixture of perlite, vermiculite, and soil (1:1:1). Prior to use, the mixture was tyndallized in an autoclave for one hour on three consecutive days. Seedlings were maintained in a greenhouse at 25 °C, with 70 % relative humidity and a 12:12 LD photoperiod by the use of 400 nmol.m-2.s-1 cold light lamps.
Inoculation techniques: For seed inoculation technique, 10 g of seeds were immersed in 10 ml of B. bassiana conidial suspension 0.01 % (v/v) Tween 80 (1×108 conidia ml-1) for 24 h, according to the methodology of Brownbridge et al. (2012). The seeds were left to dry on sterile filter paper in a laminar flow chamber for 30 min, then planted in pots at a 4 cm depth and maintained in a greenhouse at 25 °C with a 12:12 LD photoperiod. Control seeds were immersed in a conidial-free solution of 0.01 % Tween 80. In the case of foliar spraying, the leaves of pepper plants of 30 cm height were sprayed with 3 ml of a B. bassiana conidia suspension (1×108 conidia ml-1) using a 30 ml sterile glass manual sprayer (Posada et al. 2007). To prevent the conidia runoff into the ground, the upper part of each pot was covered with aluminium foil (Posada et al. 2007). Control plants were sprayed with a conidial-free solution of 0.01 % Tween 80. For root dipping, each seedling was removed from the pot and rinsed three times with sterile distilled water. The tips of the roots were cut for better absorption and each root seedling was placed individually in test tubes with 2 ml of B. bassiana conidial suspension (1×108 conidia ml-1), according to the methodology of Akello et al. (2007). Each tube was covered with aluminium foil to prevent damage from UV rays. The roots of control plants were immersed in sterile distilled water. Tubes with both inoculated and control seedlings were incubated at 25 °C in the dark with 80 % relative humidity for 24 h. Then, both inoculated and control seedlings were placed on sterile filter paper until they were completely dried and replanted in the same pots. The seedlings in all the treatments were irrigated as required and maintained in a greenhouse at 25 °C, with 70 % relative humidity and a 12:12 LD photoperiod. Thirty replicates and 30 controls were prepared for each inoculation technique.
Evaluation of the presence of B. bassiana, natural endophytes and antifungal activity in vitro: For each inoculation technique, the presence of B. bassiana in the seedlings was evaluated at 7, 15 and 28 days after inoculation. One leaf was chosen randomly from each plant and superficially disinfected by the immersion in 70 % ethanol for 2 min, followed by 0.5 % sodium hypochlorite for 2 min and rinsed with sterile distilled water (Arnold, Maynard, & Gilbert, 2001). Disinfection was verified by inoculating 100 ml of water from the last rinse of each sample onto a Petri dish with PDA culture medium. In addition, subsamples of surface-sterilized leaves were cultured in PDA plates to determine if the residual conidia retained the germinative potential (Gurulingappa et al. 2010). Leaves were dried on sterile paper under laminar flow chamber and their edges were cut to eliminate the dead tissue resulting from the sterilization process. For every sample six leaf fragments of approximately 1 cm2 of each of the 30 inoculated and control seedlings for each inoculation technique were used, totalizing 180 plants and 1 080 leaf fragments examined. Leaf fragments were cut using a sterile scalpel and placed onto Petri dishes with 20 mL of PDA medium with 2 mL of antibiotics (0.5 g streptomycin and 0.25 chloramphenicol/200 mL).
The presence or absence of B. bassiana in the leaf fragments was recorded after 10 days of incubation at 25 °C and expressed as the frequency of colonization (number of colonized leaf fragments/ total number of leaf fragments) × 100 (number of colonized leaf fragments = a fragment with one B. bassiana colony, Petrini & Fisher, 1986). In the inoculated leaf fragments, was also recorded, isolated and identified the endophytic fungi that naturally occurred in pepper leaves, with the purpose of determining the existence of antifungal activity among them and B. bassiana under laboratory conditions. The endophytic fungi were isolated and identified by traditional taxonomy (Domsch, Gams, & Anderson, 2007), examined with a Wild M8 stereo microscope and a Dialux 20 Leitz microscope. To evaluate the antifungal activity, in vitro tests were carried out using the dual culture method (Paul, Deng, Sang, Choi, & Yu, 2012). The species of natural endophytes and B. bassiana LPSc 1067 were individually cultured on APG medium and incubated at 25 °C for 4 days. Afterward, the agar disks (5 mm diam.) with mycelium of each species were cut and placed, one disk of endophyte and one of entomopathogen at a distance of 6 cm from each other on a Petri dish with APG culture medium. Controls were performed for each pair of endophyte B. bassiana. The antifungal activity was evaluated by measuring the distance of the inhibition zone between the endophyte and the entomopathogen using the following scale (Taechowisan, Peberdy, & Lumyong, 2003): +++ ≥ 20 mm (strong activity), ++ = 11-19 mm (moderate activity); 2-10 mm (low activity) and - = 0 mm (no activity).
Effect of B. bassiana as an endophyte on the growth, reproduction and feeding preference of Myzus persicae: Colonies of M. persicae were initiated with clones supplied by the Faculty of Agricultural and Forestry Sciences (Universidad Nacional de La Plata, Buenos Aires, Argentina). Aphids were reared on peppers plants cultivated in pots inside cages placed in a greenhouse with a cycle of 12 h light (25 °C)/ 12 h dark (20 °C). To evaluate the effect of B. bassiana on the growth and reproduction of M. persicae, young pepper leaves inoculated with the foliar spraying technique were cut seven days before the beginning of the tests. The inoculated leaves were surface-sterilized with 70 % ethanol, sodium hypochlorite and sterile distilled water (Gurulingappa et al., 2010). Subsequently, the leaves were placed individually in sterile 500 ml containers with 3 % water agar in their base. Ten third-instar nymphs of M. persicae were transferred to the surface of each leaf using a brush of fine camel hair. The containers were maintained in a culture chamber with 12 h light at 25 °C/12 h night at 20 °C. Fifteen repetitions of each inoculated and control leaves were made. Ten days after the beginning of the tests, the number of surviving adults and the number of nymphs of the next generation were recorded. The presence of the endophyte in the plants used for the bioassay was verified three days after inoculation. One leaf was chosen randomly from each plant and superficially disinfected (Arnold et al., 2001), then leaf fragments were cut and placed onto Petri dishes with PDA and antibiotics.
To evaluate the feeding preference of M. persicae, Petri dishes with moist filter paper (moist chambers) were prepared, in which two leaves of pepper of equal size were placed, one inoculated by leaf spraying three days before the beginning of the test and one non-inoculated (control). In each dish, threeadult aphids were placed at the same distance between the two leaves. After 24 h, the number of aphids that fed on each leaf were recorded. The aphids that were not on any leaf or had the same number of individuals on each leaf were not considered for the counting (Crawford, Land, & Rudgers, 2010).
Data analyses: The analysis of variance (ANOVA) parametric test of and a posteriori Tukey test were used to analyse the differences between inoculation techniques. The time the fungus remains in the plant and the interaction between the two variables, was obtained using InfoStat (2007). Before data analysis, the Shapiro-Wilk test was used to assess data normality. The frequency of colonization expressed as a percentage was angularly transformed to stabilize the variance. The significance differences between treatments was measured by F-test (P = 0.05). Differences between mortality and reproduction of adults and nymphs between leaves inoculated and controls were evaluated using paired sample t-tests (Infostat, 2007). Corrected percent mortality was calculated using Abbott’s formula (Abbott, 1925). Differences between controls and treatments in the feeding preference of aphids were evaluated using paired sample t-tests (Infostat, 2007).
Results
Evaluation of Beauveria bassiana as an endophyte: There were significant differences between inoculation techniques (F = 11.52, df = 2, 261, P < 0.0001). Leaf spraying showed the highest colonization frequency, followed by root dipping and seed immersion that showed significantly lower values. The time after inoculation did not differ significantly in the colonization of B. bassiana (F = 0.36, df = 2, 261, P = 0.6985). However, as shown in Fig. 1, the interaction between the inoculation techniques and the time after inoculation showed significant differences (F = 5.12, df = 4, 261, P = 0.0005). The highest frequency of colonization (10 %) was obtained after 7 days with leaf spraying. No endophytic colonization of B. bassiana was recorded after 7 and 14 days using seed immersion. Likewise, using leaf spraying, the endophytic colonization of B. bassiana decreased with time, but this tendency was not observed for root dipping and seed immersion techniques (Fig. 1). In all the treatments, the inoculum used showed an average viability > 95 %.
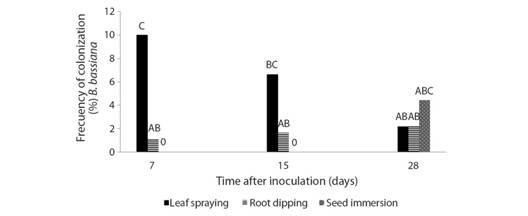
Fig. 1 Frequency ofcolonization (%) of tomato leaf fragments at 7, 15 and 28 days after inoculation with Beauveria bassiana, using different inoculation techniques (leaf spraying, root dipping and seed immersion). Bars with different letters indicate differences of statistical significance according to Tukey´s test (p < 0.05).
Natural endophytes and in vitro evaluation of the antifungal activity of B. bassiana: From the leaves of pepper inoculated with B. bassiana were isolated and identified seven species of Ascomycota anamorphs: Acremonium sp., Aspergillus terreus (Thom & Church, 1918; Aspergillaceae, Eurotiales), Chaetopsina sp., Chaetomium globosum (Kunze, 1817, Chaetomiaceae, Sordariales), Chaetomium cochliodes (Palliser, 1910, Chaetomiaceae, Sordariales), Penicillium sp. and Trichocladium pyriforme (Dixon, 1968, Chaetomiaceae, Sordariales). Leaf sprayingshowed the presenceof five species of natural endophytes with low frequencies of occurrence and the highest frequency of B. bassiana. In this treatment, Acremonium sp. was recorded with the highest frequency at 7 days after inoculation (Fig. 2A). Unlike in root dipping B. bassiana, presented a low frequency and, natural endophytes showed the greatest number of species (six species) and the highest frequencies of colonization in this treatment. As shown in Fig. 2B, C. globosum and T. pyriforme had the highest frequencies and were recorded at 7, 15 and 28 days after inoculation. Seed immersion presented the lowest number of natural endophytes with the lowest frequencies (Fig. 2C). The antifungal activity of the natural endophytes C. globosum, C. cochliodes, A. terreus, T. piriforme vs B. bassiana evaluated in vitro using the dual culture method, showed a low antifungal activity of C. globosum, C. cochliodes and A. terreus, and a moderate activity of T. piriforme against the entomopathogen.
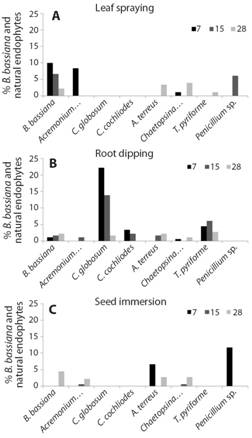
Fig. 2 Frequency of colonization (%) of B. bassiana and of natural endophytes in the pepper leaf fragments at 7, 15 and 28 days after inoculation using A. leaf spraying, B. root dipping, and C. seed immersion.
Effect of B. bassiana as an endophyte on the aphid pest M. persicae: The mortality and reproduction of adults and nymphs did not show statistical significance differences between leaves inoculated with B. bassiana and controls (adults: T = 0.75, df = 28, P = 0.45 and nymphs: T = 0.69, df = 28, P = 0.49). However, the mortality of adults tended to increase and the offspring (nymphs) to decrease in the inoculated leaves. As shown in Fig. 3, of the 150 adult aphids used for each treatment, 85 did not survive in the inoculated leaves whereas the controls had 69 dead adults. A decrease in reproduction was also observed in the inoculated leaves, with 462 nymphs in the inoculated leaves and 510 nymphs in the controls.
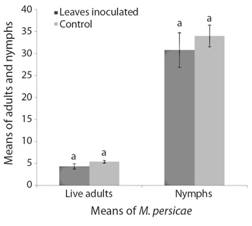
Fig. 3 Means number of M. persicae adults and nymphs recorded on inoculated and control leaves. The bars indicate SEM values (SE mean). Bars with different letters indicate differences of statistical significance according to T test (P < 0.05).
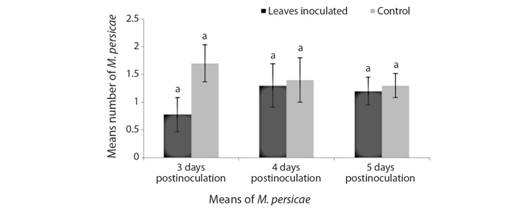
The feeding preference of the aphids did not show significant differences between inoculated and control leaves (T = 1.53, df = 58, P = 0.13). However, a greater number of individuals was observed on the control leaves. Of 30 samples analysed, 16 samples preferred control (36 aphids), 12 samples preferred inoculated (26 aphids) and 2 samples were not considered. At 3, 4 and 5 days after inoculation, a greater number of aphids was observed on the control leaves, being this difference more marked at 3 days after inoculation. The means observed at 3, 4 and 5 days after inoculation were 0.7, 1.3, 1.2 for inoculated leaves, respectively and 1.7, 1.4, 1.3 for control leaves, respectively (Fig. 4).
Discussion
Plants can harbour endophytic fungi that might induce changes in their physiology and in turn affect their interaction with herbivorous insects. Previous studies have reported the presence of different species of entomopathogenic fungi that occur as natural endophytes within a limited range of plants. In the case of B. bassiana, many studies have reported its successful inoculation and re-isolation from various plants (Tefera & Vidal, 2009), e.g. using foliar spraying in pepper (Mantzoukas & Lagogiannis, 2019), potato (Wagner & Lewis, 2000), and stem injection and foliar spraying in tomato (Allegrucci et al., 2017). However, Posada et al. (2007) observed that the leaves of some plants, such as coffee, have deficient entry routes for this fungus.
In this study, it was showed that the strain B. bassiana LPSC 1067 was established effectively as an endophyte in the pepper tissues and was re-isolated from new leaves at 7, 15 and 28 days after the inoculation using foliar spraying and root dipping. Beauveria bassiana was absent from control plants and surface-sterilized leaf washes (Gurunligappa et al., 2010). The ability of B. bassiana to establish in different plant tissues located far from the site of inoculation and its potential to move through them was evidenced by the presence of this fungus in the leaf fragments after the inoculation by root dipping and seed immersion. The most effective inoculation technique was leaf spraying and the highest colonization was recorded at 7 days after inoculation. The endophytic colonization, estimated by the frequency of B. bassiana after inoculation using this technique, decreased over time. This decrease in colonization over time might be either due to the immune response of the host towards the fungus, the expansion of leaves with the maintenance of the colonies that were already established or the possible competition with other endophytes in the plant (Posada et al., 2007).
Plants are able to recognize microbial compounds (fungal chitin) that are produced during pathogen attack and trigger defensive pathways (Zipfel, 2009; Newman, Sundelin, Nielsen, & Erbs, 2013). Beneficial fungi and saprophytic pathogens have developed adaptive mechanisms, such as the secretion of effector proteins, to facilitate the evasion of plant defences and colonize the extracellular spaces of plant tissues (Van Esse, Bolton, Stergiopoulos, de Wit, & Thomma, 2007). Schulz and Boyle (2005) described this interaction between the host plant and the endophyte as a balanced antagonism. In this study, it was observed that natural endophytes presented higher frequencies in the treatments in which the inoculated entomopathogen showed a low frequency. This might indicate the presence of antifungal activity, which interferes in the establishment of the entomopathogen in the plant tissue. Trichocladium piriforme was one of the most frequently recorded endophyte at 7, 15 and 28 days after the inoculation using root dipping, and showed a moderate antifungal activity against B. bassiana. Therefore, the presence of this endophyte in the pepper tissues appears to interfere in the establishment of B. bassiana. Evueh and Ogbebor (2008) described a significant interaction was exhibited by Trichocladium sp. in which the growth of Colletotrichum gloeosporioides was much affected by hyphal interference. The antagonistic fungus grew over the colony of C. gloeosporioides and completely inhibited its growth. Regarding the effect of the endophytic B. bassiana on the aphid pest M. persicae, we did not find significant differences in the mortality and reproduction of adults and nymphs between treatments and controls. However, the adult mortality tended to increase and the offspring (nymphs) to decrease in the inoculated leaves. These results are in accordance with similar studies performed by Clifton, Jaronski, Coates, Hodgson, and Gassmann (2018) where no significant effects on survival and reproduction of the aphid Aphis glycines were observed with B. bassiana as endophyte. On the other hand, these results contrast to those found by Mantzoukas and Lagogiannis (2019), who described a high percentage of mortality of M. persicae on pepper leaves with endophytic B. bassiana after 21 days. Probably these differences could be related to the origin of the B bassiana strain. B. bassiana strain LPSC 1067 used in this study was isolated from an orthopteran insect. There are several studies showing that strains pathogenicity is variable and has a direct relationship with the insect species host that was originally isolated (Muñoz, De la Rosa, & Toledo, 2009, Hernández Díaz-Ordaz, Pérez, & Toledo, 2010).
No differences of statistical significance were found in the feeding preference of M. persicae between inoculated and control leaves, although a greater number of aphids was observed on control leaves, being this number higher at 3 days than at 4 and 5 days after inoculation. Previous research has suggested that B. bassiana can induce plant defences, which in turn suppress insects (McKinnon, Saari, Moran-Diez, Meyling, Raad, & Glare, 2017). Future studies evaluating the indirect effects of endophytic entomopathogens on both plants and herbivorous insects are needed to further explore their potential use in the management of insect pests, as well as studies to assess the entomopathogenic activity of natural endophytes. Although the ecology and potential applied value of the specific interactions between plants, fungi, and insects have not yet been explored, the use of entomopathogens as endophytes opens interesting possibilities for the specific control of some insect pests.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio