Introduction
A wide variety of reproductive strategies and tactics in Neotropical migratory fish have ensured the adaptation and survival of their offspring in environments where biotic conditions, such as food availability and predation pressure, and abiotic conditions, such as temperature, photoperiod, and available oxygen, vary widely in space and time (Balon, 1984; Roff, 1992). The reproductive strategy used by fish species is a set of characteristics that the species must manifest in order to succeed in reproduction. Considering that each species has a distribution that is established by ecological conditions, each species must have a unique reproductive strategy (Vazzoler, 1996), which is the general reproduction pattern of individuals of that species. However, when variations in environmental conditions occur, changes in some characteristics of the strategy also occur to ensure success in the reproductive process. These variations within this pattern in response to environmental fluctuations are known as reproductive tactics (Roff, 1992; Wootton, 1998). Each reproductive strategy is a set of reproductive tactics that the species manifests to succeed over generations and ensure population balance (Winemiller & Rose, 1992; Wootton, 1998; Morgan, 2004). Understanding reproductive tactics, such as body size, weight-length relationship, sex ratio, length at first maturity, gonadal development, fecundity, gonadosomatic index (GSI), and type and timing of spawning, is essential for rational action, management and regulation of fisheries, as well as the preservation and conservation of fishery and biological stocks (Vazzoler & Menezes, 1992; Morgan, 2004; Lima, Fonteles-Filho, & Chellappa, 2007; Oliveira, Costa, & Chellappa, 2011). In addition, understanding these variables can be considered the first step in establishing patterns of life history in fish (Mazzoni & Silva, 2006).
Among the reproductive tactics that make up the reproductive biology of fish species, the sex ratio in fish may vary depending on successive events that act differently on individuals of each sex throughout the life cycle. In nature, a sex ratio of 1:1 is expected (Vazzoler, 1996), which generally occurs in more stable environments that do not suffer from frequent (and, especially, human-induced) oscillations. Sex ratio is thus an important tool for characterizing the population structure, and can be used as an indicator for the evaluation of reproductive potential and estimation of stock size (Vazzoler, 1996).
Substantial changes can be observed in fish gonads during their reproductive cycle (Nikolsky, 1963). At the same time, the weight of the gonads varies, especially in females, largely due to the accumulation of reserve material in the maturing oocytes. These changes have been evaluated using the gonadosomatic index (GSI), which expresses the percentage that the weight of the gonads represents of the total or body weight of individuals (Vazzoler, 1996; Wootton, 1998), thereby reflecting the evolution of gonadal maturation stages in the fish. The use of GSI allows determination of the reproductive period during an annual cycle.
Fish begin their reproductive cycle at a given age and length. However, it is the size or length of a fish, rather than its age, that determines the beginning of sexual maturity of the species and is a factor that is closely linked with the longevity and maximum length that the species reaches (Nikolsky, 1969; Wootton, 1998). Thus, the first maturation length (L50) has been estimated in order to determine the extent to which 50 % of individuals have already begun their reproductive cycle (Vazzoler, 1996). Knowing the value of this length is important because it is a tactic developed by the species which is closely related to growth through spatial and temporal intraspecific variations due to the prevailing environmental conditions (Vazzoler, Suzuki, Marques, & Lizama, 1997). The application of management and monitoring programs for fish stocks is based on the length at first maturity in order to prevent the exploitation of young fish and damage to spawning stocks (Froese, 2006; Vicentin, Rocha, Rondon, Costa, & Súarez, 2012).
Therefore, this study aimed to evaluate some reproductive characteristics for fish species caught in the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil. Specifically, we estimated the sex ratio, reproductive period and length at first maturity for 30 fish species. This information can serve as a reference for individuals of these species distributed throughout the Paraná River Basin, especially for studies related to functional aspects where, in most cases, this information is scarce.
Materials and methods
Study area: The Verde River Basin is a tributary on the right margin of the Paraná River, and is located to the east of the state of Mato Grosso do Sul with a drainage area of 23 739 km². Its sources are located at an altitude of 980 m.a.s.l. in the Serra das Araras Mountains in the municipality of Camapuã, central region of the state, and it runs approximately 220 km before reaching the Paraná River on the Porto Primavera Reservoir. The river comprises several rapids, small waterfalls, and narrow stretches without marginal lagoons. The main tributary of the Verde River is the São Domingos River on the left margin.
Local land use is based on farming and livestock, on which soybeans and corn are the primary crops. The industrial segment is related to livestock and includes industries such as dairy and cold stores. The landscape is divided into intensive farming areas, artificial grasslands, and natural fields with small forest, urban, and industrial areas. The vegetation in the basin primarily consists of Cerrado, with small portion consisting of Atlantic Forest. In addition, the basin has a representative area of riparian vegetation along the basin and reforestation in its South-central portion (Silva et al., 2011). On the main channel of Verde River were built one large dam, named São Domingos Dam, located in mouth of the river with the same name, and a small dam, named Verde 4 (Eletrosul Centrais Elétricas S.A., 2011). The climate and hydrological regime (Fig. 1) are characterized by two distinct seasons: dry winters (April to September) and rainy summers (October to March), similar to recorded by Silva, Gubiani, Neves and Delariva (2017).
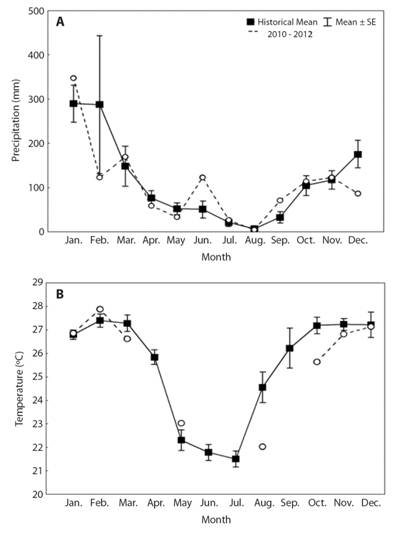
Fig. 1 Historical mean (black square) of monthly variation in A. precipitation and B. temperature, with standard errors (SE, vertical bars) for the 2005-2010 period and values recorded from 2010 to 2012 (white circles) in the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil. Historical data were provided by the National Institute of Meteorology (INMET), Três Lagoas Station.
Sampling: Sampling was performed at ten sites along a stretch of the Verde River Basin (Fig. 2), six of which were located on the Verde River, three of which were on the São Domingos River and one of which was on the Ribeirão Araras River. Sampling occurred monthly during the reproductive period of most Neotropical fish (from November 2010 to March 2011 and from October 2011 to February 2012) (Vazzoler, 1996). In addition, in order to identify atypical spawning (in the driest months), samples were taken in two months outside the reproductive period (May and August 2011 and 2012), totaling 14 months of sampling during the two annual cycles (seven samples per month; five in the reproductive period and two in the dry period). Fish were caught with gill nets (simple mesh sizes of 2.4, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14 and 16 cm between opposite knots) and trammel nets (with inner mesh sizes of 6, 7 and 8 cm between opposite knots) measuring 1.5 m in height and 20 m in length in the Verde River and 10 m in length in the tributaries. In addition to these devices, longlines with 20 hooks (size 5/0) were also used at all sampling sites, and at dusk, margin trawls (net measuring 10 m in length, 2 m in height and with a 0.5 cm mesh size between opposite knots) were operated to capture juveniles. Nets and hooks remained set for 16 h and were checked at 22:00 and 08:00.
Fig. 2 Location of sampling sites in the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil. Sampling was performed in ten sites along the Verde River Basin.
After capturing the fish, we euthanized them with a benzocaine solution (250 mg/l) following the recommendations of the American Veterinary Medical Association (AVMA, 2001), fixed them in plastic bags containing 10 % formaldehyde and placed in polyethylene containers for transport and subsequent analyses. In the laboratory, we identified the fish following Ota, Deprá, Graça and Pavanelli (2018), and measured (total and standard length in cm), weighed (total and gonad weight in grams) and eviscerated. In addition, the gender and the stage of gonadal development were identified macroscopically according to Vazzoler (1996) and Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri (2011), where the stages were classified as immature, developing, spawning-capable, regressing and regenerating. The date, location, mesh size and capture time were also recorded. Specimens of each species were preserved in 70 % alcohol and deposited in the ichthyological collection of the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia), Universidade Estadual de Maringá, available on (http://peixe.nupelia.uem.br or http://splink.cria.org.br/).
Data analysis: Reproductive metrics were estimated only for species in which the number of individuals was ˃ 30 (Table 1).
TABLE 1 Fish species and number of individuals captured in the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil, from November 2010 to August 2012
| Rank | Species | N | Rank | Species | N |
| 1 | Piabina argentea Reinhardt, 1867 | 4 568 | 45 | Parodon nasus Kner, 1859 | 16 |
| 2 | Astyanaxaff. fasciatus (Cuvier, 1819) | 1 620 | 46 | Serrasalmus marginatus Valenciennes, 1837 | 15 |
| 3 | Astyanax lacustris (Lütken, 1875) | 1 572 | 47 | *Megalonema platanum (Günther, 1880) | 11 |
| 4 | Leporinus friderici (Bloch, 1794) | 1 469 | 48 | *Pterodoras granulosus (Valenciennes, 1821) | 11 |
| 5 | Bryconamericus stramineus (Eigenmann, 1908) | 1 470 | 49 | Eigenmania virescens (Valenciennes, 1847) | 9 |
| 6 | Bryconamericus sp. | 1 283 | 50 | Megaleporinus piavussu(Britski, Birindelli &Garavello, 2012) | 8 |
| 7 | Schizodon borellii (Boulenger, 1900) | 1 086 | 51 | Hypostomus microstomus Weber, 1987 | 7 |
| 8 | Moenkhausiaaff. intermedia Eigenmann, 1908 | 669 | 52 | Hoplias sp. 3 | 7 |
| 9 | Serrapinnus notomelas (Eigenmann, 1915) | 539 | 53 | Odontostilbe cf. avanhandava Chuctaya, Bührnheim & Malabarba, 2018 | 7 |
| 10 | Megaleporinus obtusidens Valenciennes, 1837 | 519 | 54 | Serrasalmus maculatus Kner, 1858 | 7 |
| 11 | *Knodus moenkhausii (Eigenmann & Kennedy, 1903) | 340 | 55 | *Pimelodus ornatus Kner, 1858 | 6 |
| 12 | Salminus brasiliensis (Cuvier, 1816) | 280 | 56 | Schizodon altoparanae Garavello & Britski, 1990 | 6 |
| 13 | Moenkhausiaaff. sanctaefilomenae (Steindachner, 1907) | 192 | 57 | Steindachnerina insculpta (Fernández-Yépez, 1948) | 6 |
| 14 | Acestrorhynchus lacustris (Lütken, 1875) | 189 | 58 | *Loricariichthys platymetopon Isbrücker & Nijssen, 1979 | 5 |
| 15 | Pimelodella gracilis (Valenciennes, 1835) | 145 | 59 | Pimelodus maculatus La Cepède, 1803 | 5 |
| 16 | *Hemiodus orthonops Eigenmann & Kennedy, 1903 | 136 | 69 | Rhinodoras dorbignyi (Kner, 1855) | 5 |
| 17 | Galeocharax gulo (Cope, 1870) | 121 | 61 | *Sorubim lima (Bloch & Schneider, 1801) | 5 |
| 18 | Pimelodus microstoma Steindachner, 1877 | 108 | 62 | Serrapinus sp. 2 | 5 |
| 19 | Aphyocharax dentatus Eigenmann & Kennedy, 1903 | 99 | 63 | *Pimelodella taenioptera Miranda-Ribeiro, 1914 | 4 |
| 20 | Prochilodus lineatus (Valenciennes, 1836) | 96 | 64 | Sternopygus macrurus (Bloch & Schneider, 1801) | 4 |
| 21 | Hopliasmbigua Azpelicueta, Benítez, Aichino, Mendez, 2015 | 84 | 65 | Tatia neivai (Ihering, 1930) | 4 |
| 22 | Myloplus tiete (Eigenmann & Norris, 1900) | 78 | 66 | *Trachydoras paraguayensis (Eigenmann & Ward, 1907) | 4 |
| 23 | Hypostomus strigaticeps (Regan, 1908) | 73 | 67 | Crenicichla britskii Kullander, 1982 | 3 |
| 24 | Pseudoplatystoma corruscans (Spix & Agassiz, 1829) | 66 | 68 | Hypostomus cf. hermanni (Ihering, 1905) | 3 |
| 25 | *Hypostomus cf. cochliodon Kner, 1854 | 54 | 69 | Leporinus amblyrhynchus Garavello & Britski, 1987 | 3 |
| 26 | Hemisorubim platyrhynchos (Valenciennes, 1840) | 51 | 70 | Pterygoplichthys ambrosettii (Holmberg, 1893) | 3 |
| 27 | Salminus hilarii Valenciennes, 1850 | 50 | 71 | Rhamdia quelen (Quoy& Gaimard, 1824) | 3 |
| 28 | Astyanaxaff. paranae Eigenmann, 1914 | 40 | 72 | Cetopsis gobioides Kner, 1857 | 2 |
| 29 | Hypostomus regani (Ihering. 1905) | 37 | 73 | Crenicichla sp. | 2 |
| 30 | Apareiodon affinis (Steindachner, 1879) | 31 | 74 | Gymnotus sylvius Albert & Fernandes-Matioli, 1999 | 2 |
| 31 | Characidiumaff. zebra Eigenmann, 1909 | 29 | 75 | *Apteronotus ellisi (Arámburu, 1957) | 2 |
| 32 | Eigenmania trilineata López & Castello, 1966 | 27 | 76 | Schizodon nasutus Kner, 1858 | 2 |
| 33 | Rhaphiodon vulpinus Spix & Agassiz, 1829 | 27 | 77 | Cichlasoma paranaense Kullander, 1983 | 1 |
| 34 | Leporinus octofasciatus Steindachner, 1915 | 25 | 78 | *Cichla piquiti Kullander & Ferreira, 2006 | 1 |
| 35 | Roeboides descalvadensis Fowler, 1932 | 25 | 79 | *Erythrinus erythrinus (Bloch & Schneider, 1801) | 1 |
| 36 | Hoplias sp. 2 | 22 | 80 | Hypostomus cf. iheringii (Regan, 1908) | 1 |
| 37 | *Laetacara araguaiae Ottoni & Costa 2009 | 22 | 81 | Hypostomus margaritifer (Regan, 1908) | 1 |
| 38 | Auchenipterus osteomystax (Miranda Ribeiro, 1918) | 21 | 82 | Imparfinis mirini Haseman, 1911 | 1 |
| 39 | Brycon orbignyanus (Valenciennes, 1850) | 20 | 83 | Leporinus striatus Kner, 1859 | 1 |
| 40 | Leporellus vittatus (Valenciennes, 1850) | 20 | 84 | Pimelodella avanhandavae Eigenmann, 1917 | 1 |
| 41 | Leporinus sp. | 20 | 85 | Pimelodella sp. | 1 |
| 42 | Iheringichthys labrosus (Lütken, 1874) | 19 | 86 | Rineloricaria sp. | 1 |
| 43 | Leporinus lacustris Campos, 1945 | 16 | 87 | *Steindachnerina brevipinna (Eigenmann & Eigenmann, 1889) | 1 |
| 44 | Trachelyopterus galeatus (Linnaeus, 1766) | 16 | Total of individuals captured | 17 567 | |
N = number of individuals, * = non-native species.
Sex ratio: Sex ratios were calculated for each species captured in each collection period. The chi-square test (χ²) was applied to test for differences in male-female ratio, with a significance level of P = 0.05. Chi-square is a nonparametric test, which allows us to evaluate whether a set of observed frequencies is similar to those that are theoretically expected. In fish, the expected sex ratio is 1:1 (Vazzoler, 1996).
Stages of gonadal development: These stages were evaluated for the species in which males and females were captured. The data were tabulated in electronic spreadsheets and analyzed using descriptive statistics based on the frequency of each gonadal development stage. The results were incorporated into graphs to characterize the gonadal development along the year.
Gonadosomatic index: The gonadosomatic index (GSI) used to infer the reproductive period was calculated according to the equation proposed by Vazzoler (1996): GSI = (Wg / (Wt - Wg)) × 100; where Wg = gonad weight (g) and Wt = total weight (g). The gonadosomatic index was calculated only for females, since gonad weight variability is more representative in this sex than in males.
Length at first maturity: Length at first maturity (L50) was calculated only for the species that presented individuals with immature and spawning-capable gonads sufficient to fit the model. Length at first maturity was estimated by the equation Fm = 1-e-aSLb (logistic model; Fm = frequency of mature individuals; SL = standard length (cm)) (Santos, 1978). For the model fit by linear regression, the variables Fm and SL were log-transformed for linearized relations (ln[-ln(1-Fm)] = lna + b lnSL). Scatter plots were fitted for visual inspection of outliers prior to regression analysis. Extreme outliers were deleted from analyses. Analysis of covariance (ANCOVA) (Goldberg & Scheiner, 1993) was used to test for differences between parameters adjusted for males and females (species with N > 30 individuals by sex). The significance level used for all analyses was P < 0.05.
Results
A total of 17 567 individuals belonging to 87 fish species were captured (Table 1). According to our exclusion criteria, reproductive aspects were evaluated for 30 fish species (Table 2).
TABLE 2 Descriptive statistics and parameters for estimation of the sex ratio, length at first maturity and reproductive period in thirty fish species from the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil, from November 2010 to August 2012
| Order | Family | Species | Sex ratio | Length (SL; cm) | Reproductive period | ||||||||||||||||
| N | F (%) | M (%) | χ² | Min | L50 (± 95 % CI) | Max | J | F | M | A | M | J | J | A | S | O | N | D | |||
| CHARACIFORMES | Acestrorhynchidae | Acestrorhynchus lacustris | 189 | 39.7 | 60.3 | 8.1 | 5.4 | 23.2 | |||||||||||||
| Anostomidae | Megaleporinus obtusidens | 519 | 40.8 | 59.2 | 17.4 | 6.1 | 54.0 | ||||||||||||||
| Leporinus friderici | 1 469 | 64.9 | 35.1 | 131.2 | 4.4 | 38.1 | |||||||||||||||
| Schizodon borellii | 1 086 | 25.9 | 74.1 | 252.8 | 16.0 | 42.2 | |||||||||||||||
| Aphyocharacinae | Aphyocharax dentatus | 99 | 42.4 | 57.6 | 2.3 | 2.8 | 4.1 | ||||||||||||||
| Characinae | Galeocharax gulo | 121 | 58.7 | 41.3 | 3.6 | 10.0 | 23.5 | ||||||||||||||
| Cheirodontinae | Serrapinnus notomelas | 539 | 49.0 | 51.0 | 0.2 | 1.6 | 2.0 (1.5-2.6) | 2.9 | |||||||||||||
| Serrasalminae | Myloplus tiete | 78 | 34.6 | 65.4 | 7.4 | 5.3 | 27.0 | ||||||||||||||
| Characidae Incertae Sedis | Astyanax lacustris | 1 572 | 41.5 | 58.5 | 45.7 | 2.3 | 4.1 (3.2-5.3) | 13.0 | |||||||||||||
| Asyanax aff. fasciatus | 1 620 | 42.4 | 57.6 | 37.4 | 2.1 | 5.3 (3.1-9.0) | 13.5 | ||||||||||||||
| Astyanax aff. paranae | 40 | 13.0 | 27.0 | 41.0 | 3.8 | 7.5 | |||||||||||||||
| Bryconamericus stramineus | 1470 | 46.1 | 53.9 | 8.8 | 2.1 | 6.1 | |||||||||||||||
| Bryconamericus sp. 1 | 1 283 | 57.2 | 42.8 | 26.7 | 2.4 | 6.2 | |||||||||||||||
| Knodus moenkhausii | 340 | 58.8 | 41.2 | 10.6 | 2.3 | 4.4 | |||||||||||||||
| Moenkhausia aff. intermedia | 669 | 44.7 | 55.3 | 7.5 | 1.9 | 6.5 | |||||||||||||||
| Moenkhausia aff. sanctaefilomenae | 192 | 42.2 | 57.8 | 4.7 | 2.5 | 6.2 | |||||||||||||||
| Piabina argentea | 4 568 | 54.0 | 46.0 | 28.7 | 2.1 | 7.5 | |||||||||||||||
| Salmininae | Salminus brasiliensis | 280 | 43.9 | 56.1 | 4.1 | 18.0 | 29.6 (18.6-47.4) | 81.0 | |||||||||||||
| Salminus hilarii | 50 | 58.0 | 42.0 | 1.3 | 11.6 | 36.0 | |||||||||||||||
| Erythrinidae | Hopliasmbigua | 84 | 52.4 | 47.6 | 0.2 | 6.9 | 18.0 (13.7-23.7) | 36.5 | |||||||||||||
| Hemiodontidae | Hemiodus orthonops | 136 | 54.4 | 45.6 | 1.1 | 16.5 | 31.4 | ||||||||||||||
| Parodontidae | Apareiodon affinis | 31 | 64.5 | 35.5 | 2.6 | 3.4 | 11.9 | ||||||||||||||
| Prochilodontidae | Prochilodus lineatus | 96 | 40.6 | 59.4 | 3.4 | 28.0 | 47.2 | ||||||||||||||
| SILURIFORMES | Heptapteridae | Pimelodella gracilis | 145 | 61.4 | 38.6 | 7.5 | 3.5 | 18.0 | |||||||||||||
| Hypostominae | Hypostomus cf. cochliodon | 54 | 57.4 | 42.6 | 1.2 | 12.7 | 28.2 | ||||||||||||||
| Hypostomus regani | 37 | 13.5 | 86.5 | 19.7 | 6.2 | 28.0 | |||||||||||||||
| Hypostomusstrigaticeps | 73 | 28.8 | 71.2 | 13.2 | 6.0 | 25.6 | |||||||||||||||
| Pimelodidae | Hemisorubim platyrhynchos | 51 | 29.4 | 70.6 | 8.7 | 23.7 | 54.0 | ||||||||||||||
| Pseudoplatystoma corruscans | 66 | 19.7 | 80.3 | 24.2 | 53.0 | 127.0 | |||||||||||||||
| Pimelodus microstoma | 108 | 31.5 | 68.5 | 14.8 | 5.7 | 19.5 | |||||||||||||||
| Total | 17 065 | 48.5 | 51.5 | 15.1 | Number of species in reproduction | 24 | 22 | 10 | 11 | 16 | 23 | 29 | 27 | ||||||||
| Frequency % | 80.0 | 73.3 | 33.3 | 36.7 | 53.3 | 76.7 | 96.7 | 90.0 | |||||||||||||
N, total fish captured; F, females; M, males; SL, standard length; Min, minimum value reported; L50, length at first maturity; CI, confidence interval; Max, maximum value reported. Reproductive period: each letter represents one month from January (J) to December (D); yellow cells, months in which the species presented GSI values for the period; orange cells, highest GSI values for the reproductive period; gray columns, months in which there was no sampling. Significant differences for sex ratio are shown in bold (P < 0.05, chi-square test).
Sex ratio: There was a significant predominance of males (51.5 %) over females (48.5 %) (χ² = 15.10) (Table 2). For 30 species analyzed, 21 showed significant differences in sex ratio (Table 2). Sixteen species showed predominance of males: for the species Schizodon borellii, Hypostomusregani, H.strigaticeps,Hemisorubim platyrhynchos and Pseudoplatystoma corruscans, more than 70 % of captured individuals were males. Five species exhibited a higher frequency of females: among them Leporinus friderici (64.9 %) and Pimelodella gracilis (61.4 %) were the most significant (Table 2).
Reproductive period: The reproductive period ranged from two to eight months (Table 2). Approximately 70 % of the species have their reproductive period between October and February. This reproductive pattern was corroborated by the higher gonadosomatic index values, which occurred in the coincident months (Fig. 3). In addition, the highest reproductive frequencies occurred in November, December and January (96.7, 90 and 80 %, respectively), highlighting that the reproductive peak of the fish species occurs in these three months.
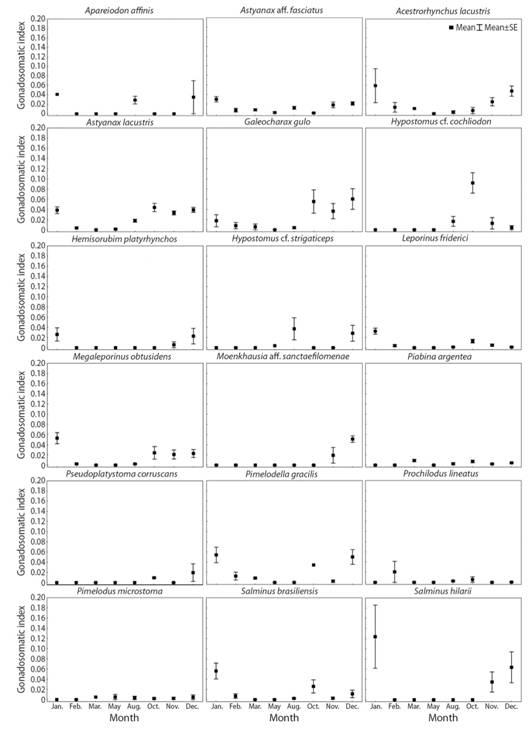
Fig. 3 Monthly variation (sampled months) of the mean gonadosomatic index (GSI) (± SE, standard error) in females of eighteen fish species captured in the Verde River, upper Paraná River Basin, state of Mato Grosso do Sul, Brazil, from November 2010 to August 2012.
Length at first maturity: Out of 30 species, it was only possible to estimate the length at first maturity for five (Table 2). Among these species, it should be noted that only one is large and of commercial interest: Salminus brasiliensis, which had a maximum length above 80 cm, and its first maturity length was 29.6 cm, with a confidence interval ranging from 18.6 to 47.4 cm.
Discussion
Understanding the various aspects of the reproductive biology of fish is a key step in implementing effective strategies for sustainable management of fisheries, as well as in defining public policies for both fisheries and aquaculture (Martins-Queiroz, Mateus, Garutti, & Venere, 2008; Chellappa, Bueno, Chellappa, Chellappa, & Val, 2009; Rondineli & Braga, 2010). In addition, to know these characteristics can allow that functional approach can be applied to achieve the measures cited above, as well as they provide many elucidating answers about species ecology (Agostinho, Benedito-Cecilio, Gomes, & Sampaio, 1994).
In this way, the sex ratio for fish is most often 1:1 for males and females. However, variations in this proportion have been observed, especially when influenced by the time of year or by unequal proportions in different length classes (Vazzoler, 1996). Our results showed higher number of males for 16 of the 30 fish species evaluated. In addition, only five species had more females than males. According to Nikolsky (1969), the population’s food supply can be considered an important interfering factor on the sex ratio. According to the same author, in oligotrophic aquatic environments, males predominate, while females predominate when available food is abundant. In this way, the conditions of food availability in the Verde River can be closer to oligotrophy (for more details see Silva, Gubiani, & Delariva, 2014) and in accordance, there was a higher proportion of males than females in most species. However, other important factors, such as predation, variations in environmental conditions, changes in birth rates or mortality of individuals of a particular sex, may also promote changes in the sex ratio in fish (Garcia, Vieira, Winemiller, & Raseira, 2004).
Intrinsic and extrinsic factors affect the reproductive dynamics of Neotropical fish (Vazzoler, 1996). Extrinsic variables, such as annual precipitation and temperature cycles, promote strong reproductive seasonality in this region. The beginning of gonadal development of different species, especially migratory fish species, occurs at a period of initial rise in temperature and precipitation, usually in October and November, which is prior to the breeding season. In addition, complete maturation occurs when environmental conditions are appropriate for fertilization and reproductive development (Vazzoler, 1996), that is, the period of maximum precipitation and temperature, which happens in February and March, months that are considered to be reproductive peaks. The environmental variables associated with the photoperiod can determine where and when each species of fish will spawn in order to ensure and maximize the development of their young (Gogola, Sanches, Gubiani, & da Silva, 2013). Neotropical fish begin their reproductive cycle in association with rising temperatures and the beginnings of precipitation and rising water levels, since more food and shelter is available during this period (Lowe-McConnell, 1999). In the Paraná River Basin, the fish reproductive cycle generally begins in October and peaks in December and January, when most species in this basin synchronize their reproductive period with higher temperatures and precipitation levels (Vazzoler, 1996). Our results corroborate the same pattern for the reproductive cycle of fish species in the Verde River, except that the reproductive peaks occurred between November and January for most species, thereby prematurely initiating this process one month early. It is also important to highlight that some species (Apareiodon affinis and Hypostomusstrigaticeps) prematurely initiated the reproductive period in August. According to Vazzoler (1996), that described the reproductive biology of Neotropical fishes, there is natural variability in the reproductive period of fish species, because annual variations can occur in order to increase their reproductive success. Thus, our results similarly showed that some fish species adapted their tactics to increase reproductive success. In addition, as noted by Gogola et al., (2013) evaluating spatial and temporal variations in fish larvae assemblages in the Ilha Grande National Park, in Brazil, the reproduction process for fishes seems to occur in association with the best environmental conditions.
Genetics and the environment determine sexual maturity in fish, which can be estimated by size and age, which are, in turn, dependent on demographic conditions (Stearns, & Crandall, 1984). The beginning of sexual maturity represents a critical transition in an individual’s life. Prior to sexual maturity, energy allocation and time use are related only to the growth and survival of individuals, but after the beginning of the reproductive process, there is a potential conflict between the use of time and resources for reproduction and/or survival (Wootton, 1998). Our results showed that the length at first maturity follows values close to those observed by other authors. For example, the estimated length for Astyanax fasciatus (Vazzoler, 1996), A. lacustris (= A. altiparanae) (Orsi, Shibatta, & Silva-Souza, 2002; Gomiero & Braga, 2007) and S. notomelas (Andrade, Campos, Langeani, & Romagosa, 2008) is close to the estimated values for other populations of these same species in the basin. However, Wootton (1998) and Vazzoler (1996) affirm that the length at first maturity may vary for the same species as a function of time, population density and environmental characteristics. As such, for Salminus brasiliensis,a highly appreciated fish species in artisanal and sport fishing (Barzotto, Sanches, Bialetzki, Orvati, & Gomes, 2015) and considered a keystone species of high economic interest in the La Plata River Basin in South America (Ruaro et al., 2019), our estimated average value (29.6 cm) for the length at first maturity was lower than that recorded by Bozza and Hahn (2010) (37.8 cm) in the upper Paraná River floodplain. It is important to highlight that, while these values are within the confidence interval estimated in our study, they can help stakeholders in the decision-making of fishery management measures, as for example with the minimum capture size of “dorado” in the Verde River Basin.
In summary, the results of this study highlight that more males than females were caught for most of the fish species evaluated, contrary to that observed in most studies. In addition, the reproductive period for fish in the Verde River Basin extends from October to January. However, we observed greater variability in the reproductive period for some species, which anticipated their reproductive period in August and the peak of reproduction in November. This variability is probably associated with adjustments on reproductive tactics of fish species to increase their reproductive success. Lastly, estimating the length at first maturity for most Neotropical fish remains a challenge, which creates difficulties in the use of protective measures to maintain fisheries and conserve fish species.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

