Introduction
The increase in global temperature endangers many species, including sea turtles. As other reptiles, sea turtles have temperature dependent sex determination at the embryonic development phase (Yntema & Morosovsky, 1982), hence an increase in global sand temperature can cause the prevalence of a single sex forcing the species toward extinction. Most studies related to sex ratios on nesting beaches already indicate a prevalence of females across most turtle species (Standora & Spotila, 1985; LeBlanc, Wibbels, Shaver, & Walker, 2012; King, Cheng, Tseng, Chen, & Cheng, 2013; Marcovaldi et al., 2014; Tomillo et al., 2014; Tanner, Marco, Abella-Perez, & Hawkes, 2019). However, spatial and temporal variation in environmental conditions lead to changes in female production (Marcovaldi et al., 2014) like the difference in females proportion depending on the nesting beach origin found in the Great Barrier Reef in Australia (Jensen et al., 2018). These differences can be important for sea turtle conservation actions like relocation of nests or shadow management.
In Cuba, the first temperature studies were conducted for the Western region of the Cuban archipelago which reported five years of data for the Guanahacabibes Peninsula and San Felipe Archipelago (Ricardo, Muro, Bretos Trelles, & Abraham, 2013). Subsequently, Gerhartz-Muro et al., (2018) extended the study carried out in San Felipe, covering a greater number of nests and three species of turtles. Both studies found that most of nest temperatures were above 29.5 ºC, which constitutes the upper limit of pivotal temperatures (incubation temperature at which equal percent of males and females are produced during the thermo sensitive period of embryonic development) described for most populations of sea turtle species (Mrosovsky, Kamel, Rees, & Margaritoulis, 2002) and would produce predominantly female hatchlings.
Hence, is essential to continue the monitoring of the nest temperature and sex ratio of hatchlings in Cuban nesting areas, specially, when climate change is continuously changing the nesting environment of sea turtles (Fuller et al., 2013). These are key elements to carry out conservation measures that contribute to the survival of the species. Therefore, the aims of this study are to evaluate the trend of incubation temperature and the incubation period, as well as to estimate the sex ratio of Chelonia mydas (Linnaeus, 1758) hatchlings at Antonio and La Barca beaches between 2012 and 2018.
Materials and methods
Study area: The work was carried out at Guanahacabibes Peninsula, one of the most important sea turtle rookeries in southwestern Cuba (Moncada-Gavilán, Nodarse Andreu, Azanza Ricardo, Medina, & Forneiro Martín-Viaña, 2011) from the year 2012 to 2018. Two of the nestings beaches located on the south coast of the peninsula were selected: Antonio (21°54’00” N & 84°39’36” W) and La Barca (21°51’00” N & 84°45’36” W). Monitoring of the temperature started in July when the pick of the nesting season occurs. Nest analysis was done in late August or September depending on the incubation duration.
Data collection: Only nests laid in July were considered in this study to prevent intra-seasonal variation. Sex ratio was estimated from the incubation temperatures (Yntema & Mrosovvsky, 1982) and the incubation duration calculated as the number of days between the night of laying and the night when the first hatchlings emerged (Godley, Broderick, Glen, & Hays, 2002). However, taking into account that several authors suggest to subtract a different number of days from the emergence day (4 days, Hendrickson 1958; 6 days, Candan & Kolankaya, 2016; 7 days, Carr & Ogren 1960), we determined the difference between the moment of hatching recorded with the data loggers and the moment of the emergence of hatchlings recorded at the beach. The average difference (4 days) was subtracted to all the incubation periods determined at the beach and this new value was used to estimate sex ratio. For measuring temperature, two types of data loggers were used: HOBO U12 Outdoor / Industrial 4 External Channel Logger devices and HOBO Pendant® Temperature / Alarm Data Logger 8K-UA-001-08 (Onset Computer Corporation, Bourne, MA, USA), which measured the temperature only in the middle of the incubation chamber (Table 1).
TABLE 1 Number and type of data loggers used to measure temperature within Chelonia mydas nests at Antonio and La Barca beaches, 2012-2018 nesting seasons
| Nesting season | HOBO U12 | HOBO Pendant | Total data loggers* / Total nests per beach | |||
| Antonio | Barca | Antonio | Barca | Antonio | Barca | |
| 2012 | - | 4 | - | - | - | 4/70 |
| 2013 | - | - | 4 | 8 | 4/59 | 8/120 |
| 2014 | 2 | - | 4 | 9 | 6/11 | 9/29 |
| 2015 | - | 2 | 6 | 8 | 6/22 | 10/109 |
| 2016 | - | 1 | 4 | 5 | 4/12 | 6/35 |
| 2017 | - | - | 4 | 6 | 4/72 | 6/106 |
| 2018 | - | - | 5 | 7 | 5/15 | 7/27 |
* = Number of data loggers varies because some had a malfunction or were dug out by other turtles.
Each sensor was programmed to measure the temperature every two hours synchronously. Temperature data was downloaded with the software HOBOware Pro version 3.2.1 (Onset Computer Corporation, Bourne, MA, USA). Incubation period was divided in three equivalent periods and the middle one was considered as the sex-determining period (SDP). Mean daily temperatures within the SDP were used to compare nest temperatures between years.
Estimation of the sex ratio: The pivotal temperature is the developmental temperature for which the sex ratio of hatchlings is 1:1 and varies among species of turtles and between different populations. Because the pivotal temperature for Cuban nesting populations of C. mydas has not been determined, we used one described by Godley et al., (2002) for the Ascension Island population: Female ratio = 1 / (1 + exp ( - (T - 28.8) / 0.6)). For the incubation duration, a modification of the equation described by Godley et al., (2002), Female ratio = 1 / (1 + exp ( - (61.4 - ID) / 3.9)), was used together with an equation obtained from adjusting Candan and Kolankaya (2016) results to a logistic function: Female ratio = 0.002316746 + 0.935123012 / (1 + exp ( - (ID - 53.81444353) / -3.171643756)), to estimate female ratio with our incubation durations. Then, the best fit logistic equation for all this data was obtained (Fig. 1).
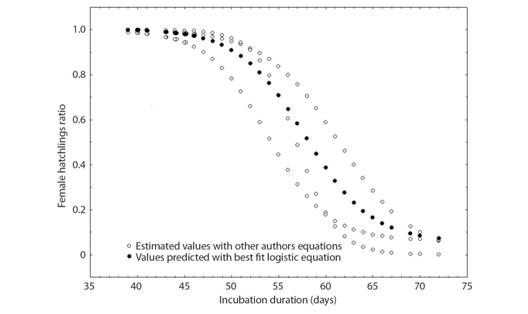
Fig. 1 Distribution of estimated values of female hatchling ratio (proportion of hatchlings that are females) according to incubation duration using the equations of Godley et al., (2002) and Candan and Kolankaya (2016) and the best fit logistic equation for all estimates (y = 0.055246883 + 0.948768615 / (1 + exp ( - (x - 57.79428483) / -3.53122753)), R2 = 0.79).
Statistical analysis: For each statistical analysis, compliance with normality assumption was verified with the Kolmogorov-Smirnov test and homogeneity of variance using the Levene test. A Kruskal-Wallis test followed by a nonparametric means comparison test were used to compare nest incubation temperatures during SDP, incubation periods and estimates of proportions of females hatchlings between beaches and years.
Results
The temperature values during the SDP have different temporal behavior for each of the beaches (Fig. 2), but 92 % of nests were above 29.5 ºC. At La Barca beach, an increase in the temperature of the nests was observed between the seasons of 2012 to 2015 (Kruskal-Wallis, P < 0.01). From 2015 to 2018, there are no differences in nest temperatures. With the exception of the year 2012, mean nest temperature was above 30 ºC. At Antonio beach, although there were differences in nest temperatures between years (Kruskal-Wallis, P < 0.01), seasons are more homogenous. The years 2013 and 2017 had the lowest temperatures in the nests (Fig. 2) while the rest had incubation temperature higher than 30 oC.
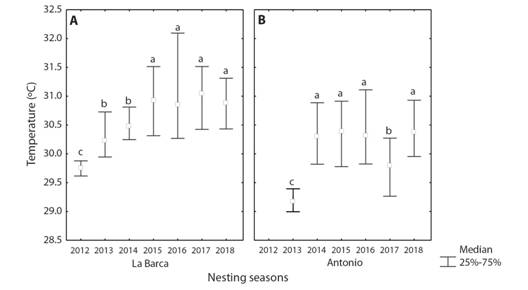
Fig. 2 Incubation temperature during the sex-determining period in Chelonia mydas nests at La Barca and Antonio beaches, Guanahacabibes Peninsula, Cuba. Lowercase letters represent statistically homogenous groups.
As a consequence of high temperatures, the incubation periods are relatively short (Fig. 3). At Antonio beach, the only difference between incubation periods was between 2013 and 2014 (Kruskal-Wallis, P < 0.01). While at La Barca beach, only 2013 was different to 2016 (Kruskal-Wallis, P < 0.05). With the exception of the nests at Antonio beach in 2013, the rest of the years at both beaches had the majority of their incubation period less than or equal to 55 days.
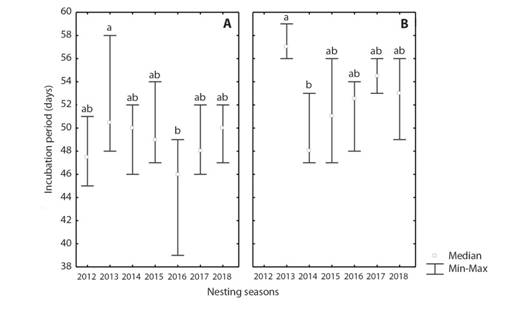
Fig. 3 Incubation duration in Chelonia mydas nests during the 2012-2018 seasons at A. La Barca and B. Antonio beaches, Guanahacabibes Peninsula, Cuba. Lowercase letters represent statistically homogenous groups.
Females hatchlings estimated production is presented in Fig. 4. Except for values estimated from temperature at Antonio during 2014 and 2017 nesting seasons, all the rest are above 85 % of female production. La Barca has particularly high female production with mean values over 95 % since 2014. It is important to highlight the consistency of both estimations, since, they lead to similar results.
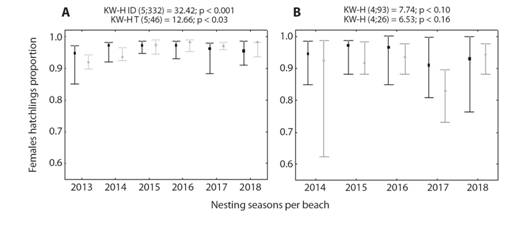
Fig. 4 Estimates of proportions of female hatchlings in Chelonia mydas nests at A. La Barca and B. Antonio beaches during the 2012-2018 seasons. Median values per season are presented, as well as the 20th and 80th percentiles (whiskers). Estimated values according to incubation duration and temperature are shown in black and gray, respectively.
Discussion
The sex ratio is a fundamental demographic parameter to achieve the self-sustainability of the populations. There are studies that historically show a predominance of females in sea turtle populations (Laloë, Esteban, Berkel, & Hays, 2016). However, the increase in global temperature, a product of climate change, may endanger the long-term persistence of some sea turtle populations because of the lack of male production (Yntema & Morosovsky 1982).
In Table 2 is presented that temperatures found during the sex-determining period at Antonio beach nests are similar to those reported by Broderick, Godley and Hays (2001), Candan and Kolankaya (2014) and Tapilatu and Ballamu (2015). However, they are cooler than those reported at Wan-an Island by King et al., (2013) and those found by Broderick et al., (2001) in Northeast Bay at Ascension Island. La Barca, on the other hand, has a wider range with values up to 33.1 oC. Therefore, they are closer to the higher values reported by Broderick et al., (2001) and King et al., (2013) which were above 33 oC.
TABLE 2 Mean temperature range during the sex-determining period in Chelonia mydas nests reported in other nesting areas
| Period | Study site | Mean range (°C) | Mean (°C) ± SD | References |
| 2013-2018 | Antonio beach, Cuba | 29.1 - 31.4 | 30.2 ± 0.6 | Present study |
| 2012-2018 | La Barca beach, Cuba | 29.1 - 33.1 | 30.7 ± 0.7 | Present study |
| 2013 | Piai y Sayang Island, Indonesia | 30.0 - 31.0 | - | Tapilatu & Ballamu, 2015 |
| 2005 | Sogözu, Turkey | 29.5 - 31.3 | 30.6 ± 0.6 | Candan & Kolankaya, 2014 |
| 2010 | Lanyu Island, Taiwan | 28.8 - 30.8 | 29.7 ± 0.7 | King et al.,2013 |
| 2011 | Wan-an Island, Taiwan | 31.1 - 33.6 | 32.1 ± 0.8 | |
| 1998-1999 | South West Bay, Ascension Island, UK | 28.7 - 31.3 | 29.5 ± 0.3 | Broderick et al., 2001 |
| 1998-1999 | Long Beach, Ascension Island, UK | 28.1 - 29.6 | 29.0 ± 0.1 | |
| 1998-1999 | North East Bay, Ascension Island, UK | 31.0-33.5 | 32.1 ± 0.2 |
According to Standora and Spotila (1985) in Tortuguero, Costa Rica, with temperatures equal to or higher than 30.3 oC, a minimum of 90 % of females occur. Most of the nests in this study have temperatures very close to or higher than this value, therefore, females hatchling production higher than 90 %, as it was estimated, can be expected.
With the exception of the years 2013 and 2017 at Antonio beach, the rest of the years for both beaches presented incubation period values similar to those reported by Broderick, Godley, Reece and Downie (2000) and lower than those reported by Godley et al., (2002) and Segura and Cajade (2010). The predominance of female hatchlings production estimated for these beaches, is consistent with most of the studies carried out for C. mydas on different nesting beaches. Spotila, Standora, Morreale and Ruiz (1987) reported 67 % of female hatchlings in Tortuguero, Costa Rica, during 1980. Mrosovsky, Dutton and Whitmore (1984) reported 67.8 % in Suriname during the 1981 season. King et al., (2013) found that of the 24 nests in which hatchlings sex was determined, 17 had more than 85 % female production, and in only three there was a predominance of males (60 - 89 %). Tapilatu and Ballamu (2015) for Piai y Sayang Island, Indonesia, refer a strongly female-skewed sex ratio while Candan and Kolankaya (2014) also reflect a predominance of females at Sogözu, Turkey. It is important to note that all the nests analyzed in this work were placed during the month of July. Therefore, the sex-determining periods of these coincided with the warmest months reported for Cuba. Hence, nests placed during the end of August and September may be contributing to a greater number of males.
The results of the present study also point out to another possible impact of increasing temperature, which is the fact of reaching sublethal or lethal temperatures leading to a decrease in the performance of hatchlings or the death of the embryos (Cavallo et al., 2015). Other studies already find a decrease in offspring viability with temperatures over 33 °C (Ackerman, 1997) or even with values between 30 - 32 °C (Tomillo et al., 2014). In summary, further studies are needed to determine actual female production in Guanahacabibes beaches and in other regions of the archipelago, as well as hatchling performance and survival at this and future thermal environments.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

