Introduction
The family Ictaluridae, or North American catfish, contains 51 nominal species, of which 29 and 10 taxa belong to the genera Noturus and Ictalurus, respectively (Page et al., 2013). Several morphotypes of native Ictalurus catfish are present in the northern Sierra Madre Occidental (SMO), Mexico (Hendrickson, 1983; Varela-Romero, 2007; Varela-Romero, Hendrickson, Yepiz-Plascencia, Brooks & Neely, 2011). Of these, only the Yaqui catfish (Ictalurus pricei) from the Yaqui, Mayo, Sonora, Fuerte, and Casas Grandes river basins (Hendrickson, Minckley, Miller, Siebert & Minckley, 1980; Miller, Minckley, & Norris, 2005) has been formally described (Rutter, 1896; Miller et al., 2005).
The presence of native catfish in the northern SMO was first reported by Rutter (1896), who described a new species from the Yaqui River basin, Villarius pricei (currently known as Ictalurus pricei), based on specimens captured in the San Bernardino creek just south of the international border with Arizona, USA. Several authors (e.g. Hendrickson et al., 1980; Miller et al., 2005; Varela-Romero et al., 2011) have referred to the presence of at least one undescribed allopatric species of the genus Ictalurus inhabiting the drainages south of the Fuerte River basin. Recently, based on analysis of mitochondrial DNA of catfish from northwestern México, Ballesteros-Córdova et al. (2015) identified the monophyly of the Yaqui catfish (Ictalurus pricei) complex, including Ictalurus sp. from the allopatric populations of the Culiacán River and San Lorenzo River basins, as its nearest genetic relative.
In support of the recently presented genetic evidence supporting the recognition of the Ictalurus sp. from the Culiacán River basin as an evolutionarily significant unit (Ballesteros-Córdova et al., 2015), we postulate that this morphotype might represent a new species based on morphological characters that distinguish it from its nearest genetic relative, the Yaqui catfish, both of which present an allopatric distribution in the SMO.
Material and methods
Study area: The study area includes the Culiacán River and Yaqui River basins in the SMO (Fig. 1), across the states of Durango, Sinaloa and Chihuahua. The Culiacán River basin provides drainage to a surface area of 19 150 km2 in the states of Durango, Sinaloa and Chihuahua, along with the Humaya (11 363 km2) and Tamazula (3 657 km2) sub-basins. For the purposes of the present study, we considered the Humaya and Tamazula sub-basins as hydrological and physiographically different in their middle and upper sections, in which large artificial reservoirs (the Adolfo López Mateos and Sanalona reservoirs, respectively) have been built for agricultural irrigation in the Culiacán coastal valley. The hot sub-humid climate in the Culiacán River basin is characterized by rains in the summer and an annual average temperature of 12-24 °C and annual mean precipitation above 600 mm (Arriaga et al., 2000). The Yaqui River basin comprises an area of 71 452 km2, providing drainage to the states of Sonora (74 %) and Chihuahua (21 %) in Mexico, and Arizona, in the USA (5 %) (Cruz-Medina & García-Páez, 2008), while its climate is controlled regionally by means of its latitudinal position, physical size, altitude, and distance to the sea. Most of the basin comprises extra-tropical drylands with a band of extra-tropical highlands that follow the SMO. The annual mean temperature along the coast and the basin inland is above 25 °C, while this is less than 15 °C in the plateau areas (Hendrickson et al., 1980).
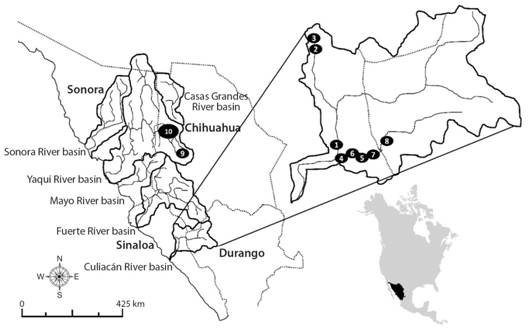
Fig. 1 Collecting sites for Ictalurus sp. (1-8) and I. pricei (9, 10) in the Río Culiacán and Río Yaqui basins, respectively, in the Sierra Madre Occidental, Mexico. Numbers indicate the collecting sites as described in Digital Appendix 1.
Fish sampling: The catfish specimens were collected during various field expeditions (1990-2012) to remote sites in the SMO, in the states of Chihuahua, Sinaloa and Durango, with access to which made difficult and risky by the presence of drug traffickers (Fig. 1, Digital Appendix 1). Sampling was carried out in two sub-basins of the Culiacán River (the Humaya and Tamazula sub-basins) and one sub-basin of the Yaqui River (the Sirupa-Tutuaca sub-basin).
Specimens were captured using AC Smith-Root 15-B POW electrofishing equipment, hooks and lines, and cast nets. In the field, the recently captured specimens were photographed for the description of their live/fresh coloration, after which fin tissue clips of each specimen were taken and preserved in 95 % non-denatured ethanol. All the specimens were fixed in 10 % formalin, preserved in 70 % ethanol, and finally donated to the fish collections of the Universidad Autónoma de Baja California (UABC), the Universidad de Sonora (USON) and the Texas Natural History Collections (TNHC) at the University of Texas.
Morphological comparison: Fifty-two Ictalurus sp. specimens (Culiacán River basin, 66-215 mm standard length [SL]) and 24 Ictalurus pricei specimens (Yaqui River basin, 186-242 mm SL) were examined for morphometric and meristic comparison. The meristic analysis was based on the standardized method of Hubbs and Lagler (1958) for the dorsal, anal, caudal, pelvic and pectoral fin-ray counts. The morphometric analysis comprised 40 somatic distances (Fig. 2A, Fig. 2B, Digital Appendix 2) following Hubbs and Lagler (1958), Bookstein et al. (1985), and Ruiz-Campos, Lozano-Vilano and García-Ramírez (2009). All linear measurements were made in millimeters (mm) along the left side of each specimen using a digital caliper (precision, 0.01 mm) connected to a PC.
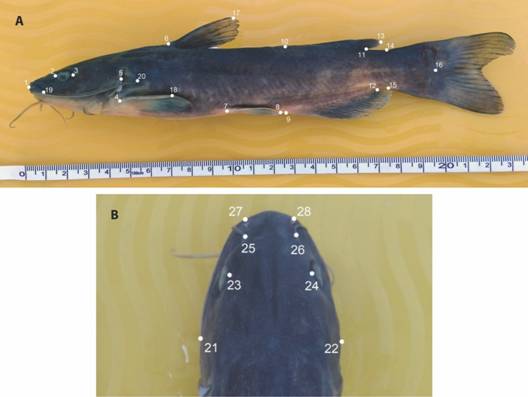
Fig. 2 Morphometric landmarks in specimens of the genus Ictalurus from the Sierra Madre Occidental, Mexico. For a description of landmarks and distances in A and B see Digital Appendix 2.
A scatterplot was used to perform an exploratory analysis of the relationship between the standard length and each morphometric character (length and width measurements) of the examined specimens. This procedure enabled the detection of any aberrant or inconsistent data in the body measurements.
Considering that morphometric variation among individuals should be attributable to body shape differences and not the relative size of the fish, an allometric method (Elliott, Haskard and Koslow, 1995) was used to remove size-dependent variation in the morphometric characters and homogenize their variances (Jolicoeur, 1963).
This allometric method enabled the standardization of the morphometric data by means of the equation Ms = Mo (Ls/Lo)b, where: Ms = standardized measurement; Mo = original length measurement of the character (mm); Ls = overall (arithmetic) mean standard length (mm) for all individuals from all populations examined; and, Lo = standard length (mm) of specimen. Parameter b was estimated for each character from the observed data using the non-linear equation, M = a Lb, where: M = the estimated measurement of the character; parameters a and b are the intercept and slope, respectively; and, L= standard length of the specimen (mm). Parameter b was estimated as the slope of the regression log Mo on log Lt, using all fish.
Three groups were considered in the DFA, two Ictalurus sp. groups (Humaya and Tamazula river sub-basins) representing the Culiacán River basin, and one I. pricei group representing the Yaqui River basin. The standardized measurements and meristic data for the catfish groups studied were compared by means of a forward stepwise DFA using Statistica 6.0 (StatSoft, Inc., Tulsa, OK, 2002). A step-by-step discrimination model is built in this type of analysis, which, at each step, reviews all variables and identifies those which contribute most to the discrimination between groups.
The statistical significance of the discrimination among populations was determined using Wilk’s lambda (λ), which oscillates from 0.0 (perfect discrimination power) to 1.0 (absence of discrimination power). The standardized coefficients of the canonical variables were determined in order to estimate the contribution of each variable in each canonical function; therefore, the value of each coefficient indicates the power of the variables considered in the analysis to either separate or discriminate.
The standardized coefficients were plotted on a Cartesian coordinate system, where their position and orientation (vectors) indicated the degree of association between each variable and the groups (Pires-Da Silva, Imhoff, Giarola & Tormena, 2001). Finally, to illustrate the separations and relationships among the populations compared, we built tree diagrams based on the squared Mahalanobis distances of the morphological characters examined.
Results
From the morphological comparison between Ictalurus sp. specimens from the Culiacán River basin (considering the Humaya and Tamazula river sub-basins separately) and Ictalurus pricei specimensfrom the Yaqui River basin, 27 of 45 the morphological variables examined entered into the forward stepwise DFA. The global Wilks lambda (λ) was 0.02701(P < 0.0001), indicating a high degree of discrimination among the groups compared. The five most significant variables included body depth at origin of anal fin (P < 0.001), number of pelvic rays (P < 0.001), distance from the orbit to the upper operculum opening (P < 0.001), number of pectoral rays (P < 0.01) and interorbital width (P < 0.05; Table 1).
TABLE 1 Lambda values of Wilks (p) and tolerance for 10 somatic variables (P ≤ 0.05) in the forward stepwise discriminant function analysis for Ictalurus sp. of the Río Culiacán basin (Río Humaya and Río Tamazula sub-basins) and I. pricei of the Río Yaqui basin, in northwestern Mexico
| Variable | Wilks | Partial | F-remove | P-level |
| Number of anal rays | 0.032422 | 0.832940 | 4.713308 | 0.0136 |
| Number of pectoral rays | 0.033093 | 0.816069 | 5.296575 | 0.0084 |
| Orbit to upper opercul opening | 0.036523 | 0.739425 | 8.281441 | 0.0008 |
| Dorsal spine length | 0.032346 | 0.834901 | 4.647038 | 0.0144 |
| Number of pelvic rays | 0.036694 | 0.735977 | 8.43033 | 0.0007 |
| Orbit length | 0.032235 | 0.837783 | 4.550237 | 0.0156 |
| Interorbital width | 0.032578 | 0.828954 | 4.848997 | 0.0122 |
| Body depth (at anal fin origin) | 0.037003 | 0.729829 | 8.699308 | 0.0006 |
| Pelvic fin origin to anal fin origin | 0.031090 | 0.868643 | 3.553706 | 0.0365 |
| Width of premaxillar dentary plate | 0.030874 | 0.874715 | 3.365901 | 0.0430 |
Number of variables in the model: 27; Wilks Lambda: 0.02701; approx. F(54, 94) = 8.8519, P < 0.0001.
Analysis of the standardized coefficients for canonical variables revealed that canonical roots 1 and 2 explained 85.6 and 14.4 % of the observed total variation, respectively (Table 2).
TABLE 2 Standardized coefficients for canonical variables resulting from the discriminant function analysis of morphological data in Ictalurus sp. of the Río Culiacán basin (n = 52) and I. pricei of the Río Yaqui basin (n = 24), in the Sierra Madre Occidental, Mexico
| Variable | Root 1 | Root 2 |
| Number of anal rays | -0.73818 | 0.25144 |
| Number of pectoral rays | -0.47715 | -0.61656 |
| Longest maxilar barbel length | -0.32428 | -0.61145 |
| Orbit to upper opercule opening | -1.04841 | -0.25635 |
| Length of caudal middle rays (from hypural plate) | 0.33992 | -0.26116 |
| Dorsal spine length | -0.13743 | -1.05172 |
| Longest lateral barbel length | -0.05913 | 0.59828 |
| Dorsal fin origin to pectoral fin origin | 0.30900 | -0.3084 |
| Number of pelvic rays | -0.62839 | 0.58116 |
| Orbital length | 0.13826 | -0.70431 |
| Pectoral spine length | 0.05883 | 0.96372 |
| Longest nasal barbel length | 0.23043 | 0.22421 |
| Interorbital width | 0.54342 | -0.84529 |
| Pectoral fin origin to pelvic fin origin | 0.19634 | -0.36921 |
| Mouth width | -0.34814 | 0.58001 |
| Body depth (at anal fin origin) | -1.08698 | -0.31499 |
| Head width (at opercules) | 0.55541 | -0.19311 |
| Pelvic fin origin to anal fin origin | -0.5702 | 0.04686 |
| Width of premaxilar dentary plate | -0.58649 | -0.22642 |
| Predorsal length | 0.21914 | 0.53912 |
| Caudal peduncle depth | 0.48098 | 0.32647 |
| Length of penultimate anal ray | -0.26833 | -0.13646 |
| Pelvic fin origin to posterior connection of adipose fin | 0.49138 | -0.19764 |
| Snout width (at maxilla) | 0.58904 | 0.06044 |
| Anal fin base length | -0.42307 | 0.36902 |
| Caudal peduncle length | -0.34173 | 0.41168 |
| Adipose fin base to caudal fin | 0.12610 | -0.39151 |
| Eigenval | 11.56908 | 1.94604 |
| Cum. Prop. | 0.856010 | 1.00000 |
Variables exerting major contributions to the observed total variation are depicted in bold.
For Canonical Root 1, four variables exerted the major effects: body depth at origin of anal fin origin (Y = -1.08698); distance from orbit to the upper operculum opening (Y = -1.04841); number of anal rays (Y = -0.73818); and, number of pelvic rays (Y = -0.62839). For Canonical Root 2, two variables exerted the major effects, namely the length of dorsal and pectoral spines (Y = -1.05172 and 0.96372, respectively).
The highest squared Mahalanobis distance (59.10) was registered between the Humaya River sub-basin and the Yaqui River basin, while the lowest (15.76) was between the Humaya River and Tamazula River sub-basins. The Tamazula River sub-basin and the Yaqui River basin recorded a distance of 39.60. The tree diagram resulting from the squared Mahalanobis distances (Fig. 3A) indicates a high degree of discrimination between the Ictalurus sp. specimens from the Humaya River and Tamazula River sub-basins (Culiacán River basin) and the I. pricei specimens from the Yaqui River basin. The Ictalurus sp. specimens from the two sub-basins of the Culiacán River revealed a high similarity level, with a value of 84.2 % (Fig. 3A).
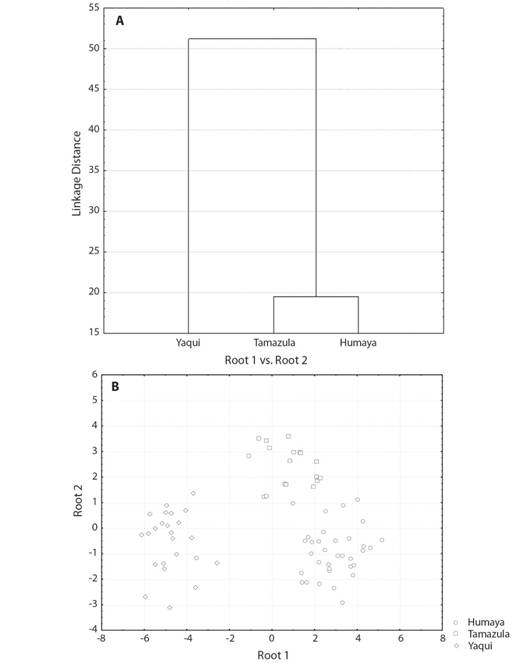
Fig. 3 (A) Tree diagram derived from the squared Mahalanobis’ distances and (B) scatter plot of centroids for roots 1 and 2 in Ictalurus sp. from the Río Culiacán basin (Río Humaya and Río Tamazula sub-basins) and I. pricei from the Río Yaqui basin, in the Sierra Madre Occidental, Mexico.
The percentage of correct classification, by means of the DFA, of individuals in the examined groups was 98.7 %, indicating that almost all individuals across the different drainages were correctly classified into their respective groups (see scatterplots in Fig. 3B). Only one individual from the Tamazula River population was classified incorrectly into the Humaya River population. In the scatterplot graph for roots 1 and 2 (Fig. 3B), the populations of the Humaya River and Tamazula River sub-basins appear as juxtaposed groups, while the population from the Yaqui River basin is widely separated from both populations of the sub-basins of the Culiacán River.
Morphologically, the Culiacán River catfish differs from the Yaqui catfish by means of the following characters: a lower number of anal (Fig. 4A), pelvic (Fig. 4B) and pectoral fin rays (Fig. 4C); smaller dimensions for head length (Fig. 4D) and predorsal length (Fig. 4E); shorter longest lateral barbel length (Fig. 4F); shorter longest dorsal ray length (Fig. 4G); narrower premaxilar dentary plate (Fig. 4H); larger distance from dorsal fin origin to last anal ray base (Fig. 4I); larger distance from dorsal fin origin to posterior end of the adipose fin base (Fig. 4J); and, finally, the tips of caudal fin lobes are rounded (Fig. 5A, Fig. 5B) while those of the Yaqui catfish are pointed (Fig. 5C).
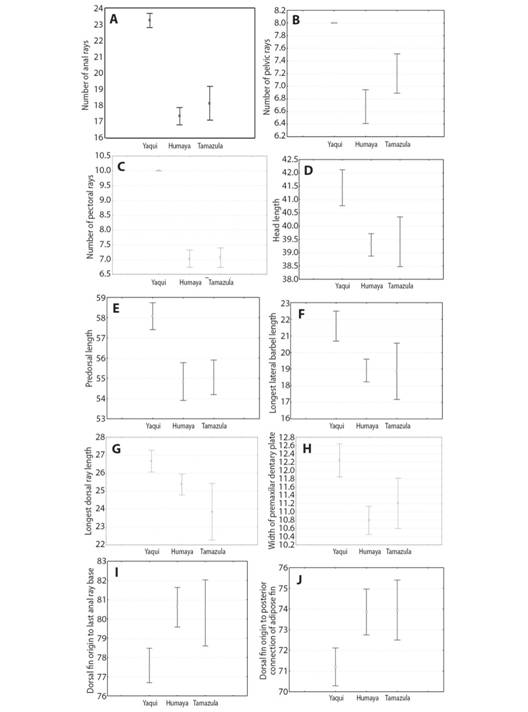
Fig. 4 Mean and 95 % confidence interval of the 10 most significant morphological characters that separate Ictalurus sp. of the Río Culiacán basin (Río Humaya and Río Tamazula sub-basins) from Ictalurus pricei of the Río Yaqui basin, Sierra Madre Occidental, Mexico.
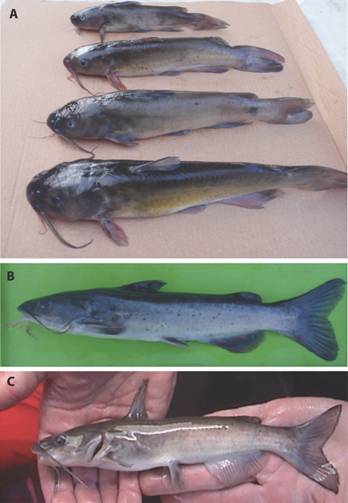
Fig. 5 Life coloration of native catfishes of the genus Ictalurusfrom the Sierra Madre Occidental, Mexico. (A) Ictalurus sp., Arroyo El Rodeo [Río Tamazula sub-basin], Durango; (B) Ictalurus sp., Arroyo Surutato [Río Humaya sub-basin], Sinaloa; and (C) Ictalurus pricei, Río Fuerte basin, Sinaloa. Photographs by S. Sánchez-Gonzáles (A and B) and A. Varela-Romero (C).
The body coloration of fresh catfish specimens from the stream El Rodeo, a tributary of the Tamazula River sub-basin (Culiacán River basin), collected on 19 February 2011 (Fig. 5A), is dark gray on the head and dorsum, while the medium gray to yellowish brown observed on the sides contrasts with the shiny white observed on the belly. Fresh catfish specimens from the stream Surutato, a tributary of the Humaya River sub-basin (Culiacán River basin), captured on 29 February 2012 (Fig. 5B), presented the following: a dark blue gray coloration on the head and dorsum which contrasts with the clear blue gray observed on the sides; small black spots are dispersed along the sides of the body and are more conspicuous on small individuals; white mental barbels contrast with the dark gray coloration of the maxillary barbels; and, all fins are dark gray. The fresh coloration of Ictalurus pricei (Fig. 5C) was clear brown on the head, dorsum and sides, while all fins had a clear gray tonality, and the caudal fin had a black edge.
Discussion
The Mexican highlands are considered a focus of biodiversity in North America (Ramamoorthy, Bye, Lot, & Fa, 1993; Bye, 1995), containing highly endemic flora and fauna as a result of the strong role played by divergence and speciation (MacCormak, Peterson, Bonaccorso, & Smith, 2008; Schönhuth et al., 2011). In the historical context of the SMO, the geographic isolation among hydrological drainages has facilitated reproductive isolation and morphological divergence, resulting in the formation of unique phenotypes (sensuWorsham, Julius, Nice, Diaz, & Huffman, 2017).
The geographical isolation of the Culiacán River catfish and its consequent differentiation (Varela-Romero et al., 2011) could be related to various orographic events and episodic aridity during the Miocene which, combined with pluvial cycles in the Pleistocene, resulted in the separation of the different hydrological drainages in the SMO (Schönhuth, Doadrio, & Mayden, 2006; Schönhuth et al., 2011, 2014; Smith et al., 2002). This tectonic-climatic event has been also proposed as the main causal factor of the speciation of other fish genera in the SMO, such as Gila (Schönhuth et al., 2014), Codoma (Schönhuth, Lozano-Vilano, Perdices, Espinosa, & Mayden, 2015) and Catostomus (Ruiz-Campos, Sánchez-Gonzáles, Mayden, & Varela-Romero et al., 2016).
Recently, molecular evidence based on cytochrome b (Varela-Romero, 2007; Varela-Romero, Ballesteros-Córdova, Ruiz-Campos, Sánchez-Gonzáles, & Brooks, in press), the cytochrome oxidase subunit 1 (Castañeda-Rivera, Grijalva-Chon, Gutiérrez-Millán, Ruiz-Campos, & Varela-Romero et al., 2014) and the sequencing of four geographical mitogenome haplotypes of Yaqui catfish and Sinaloan catfish (Culiacán River and San Lorenzo River basins) has placed the geographical haplotypes of I. pricei within a clade of specific identity. This identity is close to Sinaloan catfish haplotypes, thus supporting the hypothesis that Sinaloan catfish are a distinct evolutionary unit (Ballesteros-Córdova et al., 2015).
The discriminant function analysis applied, in the present study, on Ictalurus sp. from the Culiacán River basin (Humaya and Tamazula sub-basins) and I. pricei from the Yaqui River basin enabled the identification of a set of diagnostic morphological characters that can be used to distinguish them. The Culiacán River catfish (both sub-basins) is distinguished from the Yaqui River catfish by: its smaller average size (140 mm SL vs. 212 mm SL); lower number of anal (≤ 19 vs. ≥ 23), pelvic (≤ 7 vs. 8), and pectoral (7 vs. 10) fin rays; smaller dimensions for head length, predorsal length, longest lateral barbel length, longest dorsal ray length and premaxilar dentary plate width; and, finally, larger distances in Ictalurus sp. (Culiacán River basin) from dorsal fin origin to last anal ray base, and dorsal fin origin to posterior end of the adipose fin base.
The morphological characters described above support the hypothesis of the present study, which stipulates that the Ictalurus sp. of the Culiacán River basin (Humaya and Tamazula sub-basins) represents a different form to the nominal species Ictalurus pricei of the Yaqui River basin, as already confirmed by genetic analysis undertaken using mitochondrial DNA (Ballesteros-Córdova et al., 2015; Castañeda-Rivera et al., 2014; Varela-Romero, 2007; Varela-Romero et al., 2011; Varela-Romero, et al., in press). In this context, the Ictalurus sp. inhabiting the Culiacán River basin represents an evolutionarily significant unit (Wiley, 1981) that should be described in the taxonomic standards for future legal protection by the Mexican government (Hendrickson et al., 2003). Currently, the native catfish populations of the Culiacán River basin face several threats from anthropogenic activities, such as the alteration of their habitat by mining, the diversion or impoundment of flows, or the potential introduction of exotic catfish (Ictalurus punctatus), all of which affect the population distribution and abundance of this native taxon.
Apéndice digital 1











 uBio
uBio 

