Introduction
The traditional Chinese medicinal (TCM) plant Ecliptaprostrate, commonly known as False Daisy (lǐ cháng in Chinese), is widely distributed throughoutIndia,Nepal,China,Thailand, andBrazil.In Oriental regions, E. prostrate has medicinal usage, mainly as purgative, and external usage for skin diseases. Moreover, the decoction of E.prostrate leaves is used for normal hair-growth of new born babies in India. Research studies showed that extracts from E.prostrate can protect normal human bronchial epithelial (NHBE) cells from cigarette smoke extracts (CSE) (Ding et al., 2014); a natural compound α-terthienylmethanol from E. prostrate can induce apoptosis in human endometrial cancer cells by ROS production via NADPH oxidase (Lee et al., 2015). Recently, another natural compound Wedelolactone, isolated fromE.prostrate, exhibited prevention of vascular remodeling and neointimal hyperplasia by VSMC proliferation through Akt inhibition and AMPK activation (Peng et al., 2017).The recent interests of medicinal chemists in this plant have influence on the identification system of this plant species, and their genetic authentication from other highly morphological similar substitutes, such as Penthorum chinense Pursh (P. chinense) with different medicinal properties and activities.
P. chinense, also known as ‘Gan huang cao’, is a perennial herb with a rich history of medicinal use in different parts of China, especially among Miao ethnic group (Mei et al., 2017a; Mei, Khan, Zhang, &Fu, 2017b). P. chinense is mainly found in East Asian regions like China, Korea, Thailand, Vietnam, Laos, Mongolia as well as some parts of Russia, and in China it is found as wild-borne in several parts including Guizhou Hunan, Jiangxi, Sichuan (Flora of China, 2017). P. chinense is useful against certain diseases like liver damage, chronic liver injury and hepatitis. Reports indicate it possess antioxidant and anticancer properties, as well as being hepatoprotective, antiviral and antidiabetic (Cao et al., 2015; Feng, Wang, Dong, & Wu, 2001; Hu et al., 2015; Huang et al., 2015; Lu, Jiang, Jiang, Zhang, & Zhang, 2012; Wang, Liu, & Feng, 2006; Wang et al., 2014; Wang, Lin, & Wang, 2015; Wang et al., 2017; Zhang, Xu, Jiang, & Jiang, 2013).
In recent biotechnological era, different types of molecular bio-techniques have been applied for genetic identification and authentication of different plants, animals and bacteria. For example, AFLP (amplified fragment length polymorphism), ISSR (inter-simple sequence repeat), RAPD (random amplified polymorphic DNA), SSR (simple sequence repeat), SCAR (sequence characterized amplified region), STR (short tandem repeat) etc. (Williams, Kubelik, Livak, Rafalski, & Tingey, 1990; Fu, Yang, Khan, & Mei, 2013; Khan, Cheng, Mei, Wei, & Fu, 2016; Mei, Zhang, Liu, Imani, & Fu, 2017c; Fu et al., 2018). A combination of RAPD and SCAR marker analysis or SCAR marker alone provides a high level of authenticity of genetic identification (Fu et al., 2015; Fu et al., 2017). Molecular analysis has been simplified in a PCR reaction using PCR primers designed based on RAPD amplicons in SCAR marker technique (Kumla, Doolgindachbaporn, Sudmoon, & Sattayasai, 2012; Fu et al., 2015). However, the genetic relationships between P. chinense and E. prostrate have not been studied yet and there is no report on SCAR marker development from E. prostrate; however, we developed P. chinensee-specific SCAR markers previously (Mei et al., 2017a).
Here in this study, the SCAR markers specific to E. prostrate were developed, which can genetically identify and distinguish the medicinal plant E. prostrate from closely related herbal P. chinense Pursh, as well as other organisms.
Materials and methods
DNA Extraction:A total of nine Penthorum chinense Purshaccessions from different regions of Chinaand one Eclipta prostrate was described previously in Table 1 (Mei et al., 2017a). Other samples, Eclipta prostrate, Canarium album (Lour.) Raeusch, Dimocarpus longan Lour., Litchi chinensis Sonn., Mentha haplocalyx Briq., Lycium barbarumL., Angelica sinensis (Oliv.) Diels, Ginkgo biloba L., Herba Acroptili Repentis, Ganoderma lucidium (Leyss.ex Fr.) Karst. , Ganoderma japonpcum, Gardenia jasminoides Ellis, Artemisia argyi H. Lév. and Vaniot, were also collected from different parts of China. Genomic DNA was isolated from fresh leaves of the collected plants by using modified Cetyl Trimethyl Ammonium Bromide (CTAB) method, and the DNA quality was checked by agarose gel electrophoresis (0.8 %) and spectrophotometry (Fu, 2012; Fu et al., 2013). The plants were carefully identified and the specimens have been deposited at the source bank of the Southwest Medical University.
TABLE 1 Sources of RAPD samples
| No. | Name | Sources | Sample |
| 1 | Penthorum chinense Pursh | Yingtan, Jiangxi | YT |
| 2 | Penthorum chinense Pursh | Anqing, Anhui | AQ |
| 3 | Penthorum chinense Pursh | Ankang,Shanxi | AK |
| 4 | Penthorum chinense Pursh | Chenzhou, Hunan | CZ |
| 5 | Penthorum chinense Pursh | Luzhou, Sichuan | LZ |
| 6 | Penthorum chinense Pursh | Xichang, Sichuan | XC |
| 7 | Penthorum chinense Pursh | Zunyi,Guizhou | ZY |
| 8 | Penthorum chinense Pursh | Yichang,Hubei | YC |
| 9 | Penthorum chinense Pursh | Xinzhou,Shanxi | XZ |
| 10 | Eclipta prostrate | Luzhou, Sichuan | LZ |
Improved RAPD Amplification: Different DNA samples were used for PCR amplification with RAPD primers A11, and N7. The PCR reaction system of 10 μl was consisted of 1.5 μl genomic DNA, 5 μl 2×Taq PCR MasterMix, 1 μl 2.5 μM primer and 2.5 μl ddH2O. Applied Biosystems Veriti® 96-Well Thermal Cycler (Life Technology, USA) was used for PCR amplification, and the steps were as follows: (1) 90 s at denaturation at 95 °C for 90 s, (2) 40 cycles of denaturation at 94 °C for 40 s, annealing at 36 °C with the RAMP rate from annealing to extension adjusted to 0.125 ºC/s (5 % ramp rate) for 60 s, extension at 72 °C for 90 s, and (3) a final extension step at 72 °C for 5 min (Mei et al. 2015a, 2017).
Electrophoresis by agarose gel: PCR products for RAPD amplification were loaded for electrophoresis into agarose gel (1.5 %). The amplified PCR products for developing SCAR markers were separated into 1.8 % agarose gel. Ethidium bromide (EtBr) staining was used for visualizing the gels and the images were captured in Chemi Doc XRS system (Bio-Rad, USA) (Fu, 2012).
Molecular cloning: The bright bands in agarose gel were cut and purified (with TIANgel Mini DNA Purification Kit (DP209, Tiangen reagents, Beijing, China). The vector pGM-T (No. VT202) (purchased Tiangen reagents, Beijing, China) was sued for AT cloning and the purified DNA fragments were ligated. Then they were transformed into DH5α E. coli competent cells, and spread on LB agar plates containing ampicillin (100 μg/μl), X-gal (40 mg) and IPTG (160 μg). The plates were then incubated at 37 °C for overnight, and the white colonies were screened out by blue white screening. The correct insert was confirmed by PCR, where T7/SP6 primer pairs were used. The colony PCR condition was as follows: (1) initial denaturation at 95 °C for 90 s, then (2) 35 cycles of denaturation at 94 °C for 40 s, annealing at 60 °C for 30 s, extension at 72 °C for 90 s, and (3) a final extension step at 72 °C for 5 min, with “Applied Biosystems Veriti® 96-Well Thermal Cycler”. PCR products were then run on 1.5 % agarose gel for electrophoresis (Mei et al., 2015a; Khan et al., 2016; Fu et al., 2017).
DNA sequencing and bioinformatics: The positive clones N7-11 (clone 1) and A11-21 (clone 1) were performed sequencing by Sanger method using SP6 of T-vector primer. The homology of sequenced DNA clone was searched in BLAST (online program; available in http://www.ncbi.nlm.nih.gov/BLAST/) in GenBank database to verify the novelty of cloned fragments.
SCAR primer design: Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/) was used for designing primer pairs for SCAR based on the sequence of RAPD fragments (Cheng et al., 2019). The quality of each pair primers was tested to optimized amplification condition. Primer sequence, optimum PCR condition and PCR product length are presented in Table 2.
TABLE 2 Sequences of SCAR primers, PCR product size and PCR condition
| SCAR | 5’-primer | Sequence (5’-3’) | 3’-primer | Sequence (5’-3’) | Size (bp) | Tm (°C) |
| A11-21 | A11-21L | ATCGATTCCCGGTCCTACAG | A11-21R | TGGGTATTCTTCTTAGCAACCAA | 249 | 64 |
| N7-11 | N7-11L | AGAAAATGCAAACCGGAGGT | N7-11R | CGTTGACTCCCACACATCCT | 214 | 64 |
Species-specific SCAR markerdevelopment: For developing the stable SCAR markers, the PCR was performed with 22 DNA samples. The content of 10 μl PCR reaction was as follows: 5 μl 2×Taq PCR MasterMix, 1 μl of 2.5 μM each pair of SCAR primers, and 1μl (10 ng) genomic DNA, 3 μl of ddH2O. PCR reaction was performed in a thermal cycle (mentioned above) with the following steps: (1) an initial pre-denaturation for 90 s at 95 °C, (2) followed by 29 cycles of denaturation at 94 °C for 40 s, annealing at 64 °C for 30 s, and extension at 72 °C for 40 s, and finally (3) extension at 72 °C for 5 min.
Results
Molecular cloning of fragments generated by RAPD amplification: Results shown in Fig. 1, indicate the bands with specific primers (the white arrows). These bands were cut from the gel, and DNA was purified. A11 and N7 RAPD primers were used for the improved RAPD amplification of DNA sample Eclipta prostrate. After the PCR, ligation, the white clones were screened by PCR with SP6/T7 primer pair, and the results of colony PCR are shown in Fig. 2A. In Fig. 2A, the positive clones from fragments N7-11 showed in lane 1 and 2, and positive clones from fragments A11-21 showed in lane 3 and 4, all with expected amplified DNA-fragment sizes, whereas in Fig. 2B, both A11-21 and N7-11 also showed expected inserted fragments in lane 2 and 4 with EcoR I digestion.
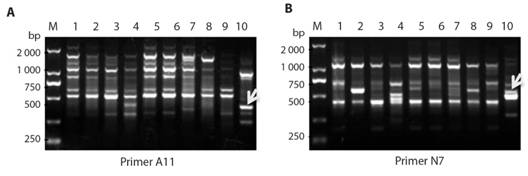
Fig. 1 Improved RAPDamplification. RAPD amplification from DNA samples from P. chinense and E. prostrate using different RAPD primers A. A11 and B. N7 (B). The arrows pointed PCR fragments were cut from gels for further cloning.
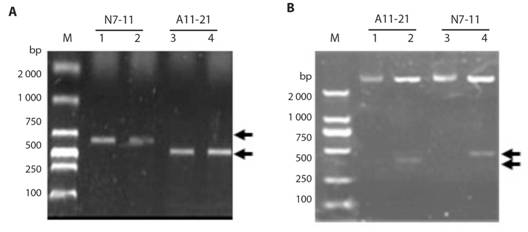
Fig. 2 Identification of positive clones after DNA ligation by colony PCR and enzymatic digestion from E. prostrate. A. Clone identification of RAPD fragments N7-11 (two clones 1, 2) and A11-21 (two clones 1 and 2). Both clones 1 in blue are selected for enzymatic digestion and Sanger sequencing. B. Clone identification of clones N7-11 (1) and A11-21 (1) by without (lanes 1,3) or with (lanes 2,4) EcoRI digestion. The black arrows represent desired PCR product or specific insert bands in different clones.
Characterization of specific fragments generated by RAPD: After the RAPD fragment clones A11-21 and N7-11 were sequenced by Sanger method, the BLAST searches indicated that the fragments were not significantly identical to any other species. Clone A11-21 consisting of 363 nucleotides, and clone N7-11 consisting of 462 were deposited into GenBank with accession number KX671034 and KX671035, respectively (Fig. 3A, Fig. 3B) Notably, the actual lengths with high quality nucleotide we obtained are shorter than that of the colony PCR or EcoR I digestion.

Fig. 3 Sequencing result of the cloned nucleotides. A. The sequences of clone A11-21 with 363 bp. The GenBank accession number: KX671034; B. The sequences of clone N7-11 with 462 bp. The GenBank accession number: KX671035.
Development of specific SCAR markers and authentication of E. prostrate from other species: To generate stable E. prostrate-specific diagnostic SCAR markers, two primers pairs were designed, and synthesized based on RAPD-dependent cloned fragments (Table 2), which were then used for PCR amplification of DNA materials collected from 22 samples of different species or cultivars for verifying species-specificity. As shown in Fig. 4, the PCR results indicated that the amplification products of A11-21, N7-11 with expected size were observed in sample E. prostrate only (Fig. 4A, Fig. 4B; lane 10); no amplification in other species, including P. chinense, demonstrating thatthe highly specific SCAR markers were successfully developed. Therefore, these SCAR markers A11-21, N7-11 can be used for the authentication of this medicinal plant from P. chinense and other species or cultivars.The two SCAR markers specific to E. prostrate, developed in this study are deposited in Genbank with accession no. KX671034 and KX671035.
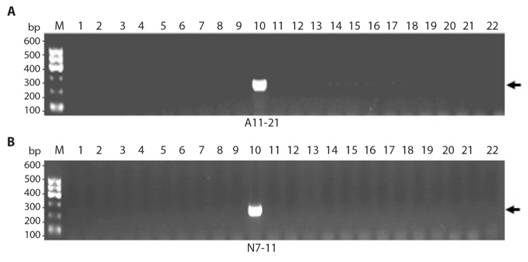
Fig. 4 Stable RAPD-SCAR marker development for A. A11-21 and B. N7-11. Lanes 1-9 are Penthorum chinense Purshes, lane 10 is Eclipta prostrate, lanes 11-22 are Canarium album (Lour.) Raeusch, Dimocarpus longan Lour., Litchi chinensis Sonn., Mentha haplocalyx Briq., Lycium barbarum L., Angelica sinensis (Oliv.) Diels, Ginkgo biloba L., Herba acroptili Repentis, Ganoderma lucidium (Leyss.ex Fr.) Karst. Ganoderma japonpcum, Gardenia jasminoides Ellis, Artemisia argyi H. Lév. &Vaniot.
Discussion
Genetic characterization and identification of numerous living organisms have been revolutionized by the development of genetic markers (Mei et al, 2017). These techniques have become very useful tools for the system biologists, botanists, zoologists, and even for the medicinal chemist. Most of these marker techniques are simple, cheap, easy to handle, and sequencing of DNA is unnecessary, but they were revealed high degrees of polymorphisms (Williams et al., 1990, Fu et al., 2013, Fu et al.,2015). Combination of RAPD with SCAR can improve the stability and specificity, and makes the process of genetic analysis more effective in studying different species (Mei et al., 2017b). Our lab and other groups, have successfully characterized a number of plant or fungal species by developing SCAR markers based on RAPD amplicons, such as, Dimocarpus longan (Yang, Fu, Khan, Zeng, & Fu, 2013), Acorus species (Ryuk, Kim, Lee, & Ko, 2014), Lonicera japonica (Yang et al., 2014), Trichoderma cf. harzianum (Pérez, Verdejo, Gondim-Porto, Orlando, & Carú, 2014), Cordyceps sinensis(Lam et al., 2015), Litchi chinensis (Cheng et al., 2015), Angelica sinensis (Zhang et al., 2015), Gardenia jasminoides Ellis var. grandiflora Nakai (Mei et al., 2015b), Ganoderma Lucidum (Khan et al., 2016), Penthorum chinense Pursh (Mei et al., 2017b), as well as developing diagnostic SCAR markers from female breast carcinomas (Fu et al., 2017).
E. prostrate is often confused with P. chinense for their phenotypes, but they are genetically different, which is confirmed by our present study. In this study, we have generated RAPD fragments by using DNA materials extracted from these plants and cloned into T-vectors by collecting E. prostrate and P. chinense species in China. Successfully, we developed two SCAR markers A11-21, N7-11, which are strictly specific to the sample of E. prostrate; not P. chinense. Thus these markers can be used for the genetic analysis, and to identify and authenticate E. prostrate from P. chinense species or other species. As the BLAST searches in GenBank database did not show similarity with these two nucleotide sequences (KX671034, KX671035), these SCAR fragments are novel molecular markers for E. prostrate species. As far we know, this is the first time to develop E. prostrate-specific SACR markers.
These E. prostrate-specific SACR markers, A11-21, N7-11, can be used for the genetic identification and authentication of this plant from P. chinense and other species or cultivars. Establishment of these SCAR markers will provide easier ways to authentically identify E. prostrate species, and contribute in the biological distinguish of this plant from P. chinense. More scientific studies can be performed to identify genomic information and isolate the active ingredients from this E. prostrate plant to investigate their medicinal properties.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

