Introduction
The soil is a fundamental component of natural and social systems, fulfilling keystone functions of biological nature such as feeding, filtering and biomechanical support. It houses various animal species responsible of the metabolic activity that are essential for its formation, functioning and fertility (Gupta & Malik, 1996). Soil organisms are the main agents of the nutrient cycles and provide essential ecosystem services for the sustainability of ecosystems, such as regulating the dynamics of organic matter (FAO, 2015).
The accelerated and sometimes irreversible degradation of soils is one of the greatest risks for humanity. Blum (2000) defines this degradation as an unbalance of soil functions, which can cause physical, chemical, and biological damage, even in some cases its destruction. In Colombia, 93 % of the agricultural soils are degraded by some level of erosion, 4.6 % are under severe erosion, and only 7 % are in good conditions (IDEAM, 2015). As an alternative practice to conserve soils properties without reducing their fertility, green manures have emerged as an agroecological technology which have proven to improve biological soil properties such as the diversity and abundance of soil invertebrates, keystone agents of soil functioning. This is of particular importance in agriculture lands owned by low-income farmers (Labrador, 2001).
Soil invertebrates are involved in different processes that allow soil functioning and its co-functioning with plants, water and the atmosphere. Much of these invertebrate species are function-specific or belong to functional groups that perform unique or a few specific functions (Stork & Eggleton, 1992; Julca, Meneses, Blas, & Bello, 2006; Culliney, 2013). Therefore, higher diversities of invertebrate species and/or its functional groups fulfill larger varieties of the several soil functions required to maintain nutrient cycling, regulation of the organic matter dynamics, carbon sequestration, transformation of the structural soil constituents, among others (Abi-Saab, 2012).
By reducing the use of traditional soil fertilizers and adopting agroecological management practices for agricultural systems can result in the colonization and/or proliferation of beneficial invertebrates for the soil (Mäder et al., 2002). The incorporation of green fertilizers is considered an agroecological practice that can improve the structure, diversity and composition of the invertebrate community associated with the soil (Guzmán & Alonso, 2008). The decomposition process of green fertilizers usually offers better environmental and nutrient conditions than traditional fertilizers, and frequently favors the colonization by diverse invertebrate species (Villalobos et al., 2000).
The aim of this research was to evaluate the effect of the application of seven green fertilizers on some biological properties of the invertebrate community in an andisol of the Nariño region in Southwestern Colombia. Due to their high fertility, the soils in this Andean region are mainly used for agricultural purposes; however, they have been severely affected by intensive cropping of potatoes, onions, peas, and other high-altitude vegetables that require chemical fertilizing for good production (Bautista, Chavarro, Cáceres, & Buitrago, 2017). This, added to the fact of low economic incomes for farmers, encourage the constant search for profitable and sustainable agroecological solutions.
Materials & methods
Location: This research was conducted at the farm “El Fondo”, 13 km far from Pasto city in the Southwestern Colombian Andes, close to the border with Ecuador. The study site locates at coordinates (77°19'57.80" W & 1°16'21.09" N), and at 2 700 msnm of altitude. The location has a temperature of 15 °C, annual rainfall of 1 527 mm and relative humidity of 70 %, has a climate is considered Dfb according to the climatic classification of Köppen-Geiger (Climate-Data.org, 2018), corresponds to a living zone according to holdridge to humid montane forest (bh-M) (Holdridge, 1996) and The soils belong to Typic Melanocryands, Lithic Melanocryands and Miscellaneous Rocky (MEBf), formed from thick deposits and thin volcanic ash over andesites. These soils have good drainage and are moderately deep to shallow (IGAC, 2004).
Experimental design: Seven species of native plants were chosen as the green fertilizers, following Prager, Sanclemente, Sánchez, Gallego, and Sánchez (2012), who suggests that these species should belong to different families, including legumes, grasses and composites. Thus, the experiment comprised seven fertilization treatments: T0: Absolute Control (no fertilization); T1: Vicia sativa L.; T2: Plantago lanceolata L.; T3: Medicago sativa L.; T4: Trifolium repens L.; T5: Brassica napus L.; T6: Lupinus mutabilis Sweet and T7: Alnus jorullensis Humboldt, Bonpland & Kunth.
These treatments were organized in a 15 × 50 m plot (750 m2) following a Randomized Complete Block design (RCB), with three replicates per treatment (24 subplots of 21.6 m2 each). The spatial distance between treatments was fixed to 0.27 m and between blocks to 1.4 m. Since the pot had a slope of 20 %, the three replicates per treatment were organized together in lines following that slope. This avoided the run-off on rainy days to mix the treatments, the experimental area commonly suffers from actions of the implementation of livestock production systems.
Sowing and incorporation of the green fertilizers: The plant seeds were obtained from Agrosemillas («Agrosemillas | Importadora y comercializadora en Medellín», s.f.), which assures germination percentages from 90 to 99 %. The following seed amounts were sown for each species: V. sativa: 166.6 g, P. lanceolata: 166.16 g, M. sativa: 154 g, T. repens: 151 g, B. napus: 141.6 g and L. mutabilis: 661.6 g. On March 15, 2018 the seeds were broadcast sown at high densities in the 24 subplots (Carreón & Martínez, 2016). After 60 days the sowing, 8 kg of leaves each of species were cut and mixed with the surface soil layer (0-10 cm) (Morán et al., 2012). From the trees near the plot, forage was collected, cutting the A. jorullensis leaves with stainless scissors and placing them in plastic bags, to be transferred to the experimental plot.
Sampling of soil invertebrates: After 45 days of the incorporation of the green fertilizers, a sampling of soil invertebrates was carried out using the TSBF methodology (Tropical Soil Biology and Fertility) described by Anderson and Ingram (1993). Three sampling points in each of the 24 experimental subplots were randomly located and a core of 0.25 × 0.25 × 0.30 m (volume = 0.0625 m3) was obtained from each one. Three subsamples were taken from each core (0-10 cm, 10-20 cm, and 20-30 cm deep) and all the arthropods and annelids were manually extracted. These three depths were considered as an additional factor for the experimental design. The arthropods were preserved in 70 % alcohol and the annelids in 5 % formalin. All the samples were transported to the Entomology Laboratory of the University of Nariño who use the taxonomic keys Doreste (1984), Fauchald (1977), Triplehorn and Johnson (2004) were identified at the family level using a Nikon smz 646 stereoscope, in each sampling carried out, the biomass was established, calculating the grams of fresh weight of the individuals per m2 in each of the treatments, by using a precision balance.
Data analysis: For each sample we measured the family richness, the abundance of individuals, biomass. A curve of family accumulation was built and used to assess the efficiency of the sampling effort. Jack 1 and Bootstrap statistics were estimated using the Estimate S version 9.1.0 program (Colwell, 2006).
The Simpson (D) and Shannon-Wiener (H) diversity indexes (Moreno, 2001) were estimated using software Past ver 3.20. Two-way analyses of variance were performed to evaluate the effect of the green fertilizer, soil depth and their interaction (independent variables), on the family richness, abundance, biomass and diversity of soil invertebrates (dependent variables). Tukey post-hoc tests with a level of significance of 0.05 were performed for each variable in order to locate the differences inferred by the analyses of variance. Finally, a type II linear regression (Reduced Major Axis) (Sokal & Rohlf, 1995) was fitted to understand the relationship between the abundance of individuals and the family richness. These analyzes were done using software R-studio 1.0.153 (RStudio Team, 2017).
Results
Abundance: The abundance of individuals for the whole experimental plot was 9 488. A. jorullensis had the highest abundance (2 080 individuals) and Control the lowest (512 individuals). Table 1 shows the total abundance for each invertebrate family under each green fertilizer treatment. The analysis of variance showed a significant effect of depth (F = 3.08, P < 0.05) and a marginally significant effect of the green fertilizers (F = 2.08, P = 0.06) on the mean abundance of individuals. Application of green fertilizers affected this abundance in a different way for each soil depth, showing higher average values in 0-10 cm in the A. jorullensis, B. napus and L. mutabilis treatments (384, 309 and 234 individuals, respectively) (Fig. 1). The Control treatment showed the lowest mean abundance, with 26 individuals. However, the largest variation in abundance occurred mainly in the surface soil layer (0-10 cm). This was conspicuous in the A. jorullensis treatment, which had a 14-fold higher mean abundance than the Control at this depth.
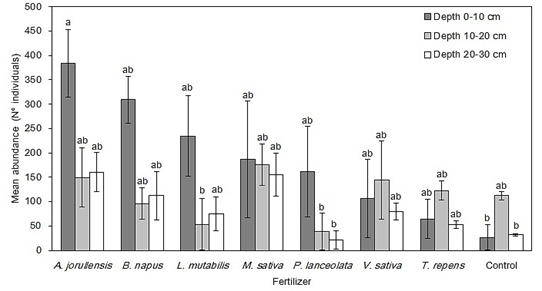
Fig. 1 Comparison of mean abundances of soil invertebrates among each green fertilizer treatment and each soil depth at the experimental plot El Fondo in Southwestern Colombia. Vertical bars represent the standard error of the mean and different letters show significant differences according to the post-hoc Tukey test (P < 0.05).
Family richness: Although the family accumulation curves did not reach a stabilized asymptote, the efficiency in the sampling effort was around 50 %, which is considered satisfactory (Fig. 2) (Chao, Colwell, Lin, & Gotelli, 2009). The analysis of variance showed a significant effect of green fertilizers (F = 3.62, P < 0.01), the soil depth (F = 8.77, P < 0.001) and their interaction (F = 2.02, P < 0.05) on the richness of invertebrate families. Green fertilizers increased the family richness differentially for each soil depth compared to Control treatment (Fig. 3). The treatments with the highest richness were A. jorullensis and B. napus; however, similarly to the results for invertebrate abundance, these variations occurred more intensely in the surface soil layer (0-10 cm). At greater depths (10-20 and 20-30 cm) the family richness also increased in some treatments, but with smaller effect sizes than in the surface soil.
Our data also showed differences in family composition among fertilization treatments, not only due to families with higher abundances, but also because invertebrate families unique for some treatments or seldomly shared among them. Very clear examples of unique treatment-invertebrate associations were M. sativa-Miridae, A. jorullensis-Tipulidae, V. sativa-Polydesmidae, L. mutabilis-Formicidae, and T. repens-Noctuidae.
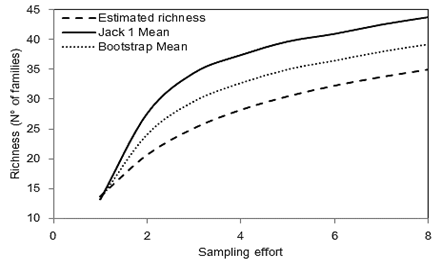
Fig. 2 Accumulation curve of soil invertebrate families at the experimental plot El Fondo in Southwestern Colombia. Statistical estimators of richness are shown as means for all the experimental subplots (Estimated richness, Jack 1 Mean, Bootstrap Mean).
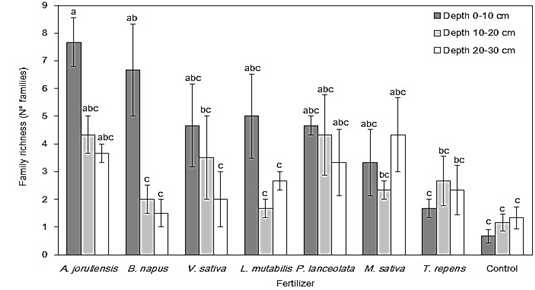
Fig. 3 Comparison of the mean family richness of soil invertebrates between each green fertilizer treatment and each soil depth at the experimental plot El Fondo in Southwestern Colombia. Vertical bars represent the standard error of the mean and different letters show significant differences according to the post-hoc Tukey test (P < 0.05).
Biomass: The analysis of variance showed a significant effect of the green fertilizers (F = 3.4, P < 0.01), soil depth (F = 3.21, P < 0.05) and their interaction (F = 2.67, P < 0.05) on the invertebrate biomass (Fig. 4). Biomass was particularly high at depth 10-20 cm in the Control treatment, with a mean value of 161.76 g. The treatments with lower biomass were A. jorullensis and T. repens, with mean values of 1.92 g and 0.69 g, respectively. In general, biomass tended to decrease in those treatments with higher invertebrate richness and abundance (Fig. 5).
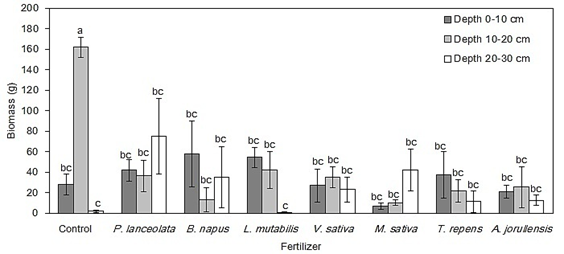
Fig. 4 Comparison of the mean biomass of soil invertebrates between each treatment and each soil depth at the experimental plot El Fondo in Southwestern Colombia. Vertical bars represent the standard error of the mean and different letters show significant differences according to the post-hoc Tukey test (P < 0.05).
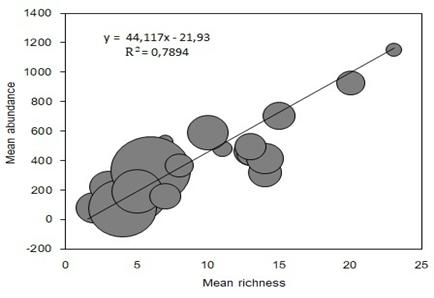
Fig. 5 Relationship between family richness and the abundance of individuals in the 24 soil cores in the experimental plot “El Fondo” in Southwestern Colombia. The line represents a type II linear regression model (Reduced Major Axis), with parameters 44.11 (slope) and -21.9 (intercept), and with R2 = 0.78, (P < 0.001). The size of the points represents a proportional measure of the biomass, standardized to the abundance registered of each core.
Diversity: The analysis of variance for the Shannon index showed a significant effect of the green fertilizers (F = 2.67, P < 0.01) and soil depth (F = 4.34, P < 0.05). For the Simpson index there was only a significant effect of the green fertilizers (F = 2.67, P < 0.05). The Shannon index was higher in the A. jorullensis treatment (H = 1.30), followed by P. lanceolata (H = 1.15), while the lowest value was found for the Control treatment (H = 0.39). The highest Simpson index was found for Control (D = 0.75) and T. repens (D = 0.67), while the A. jorullensis treatment had the lowest value (D = 0.39) (Fig. 6). These results show that the application of green fertilizers reduced the dominance of few families (Lumbricidae in the case of Control), but increased the number of families present in the soil, as well as the prerational evenness of abundance among them. This last was the case of A. jorullensis treatment, colonized by 20 invertebrate families at the end of the experiment (Table 1).
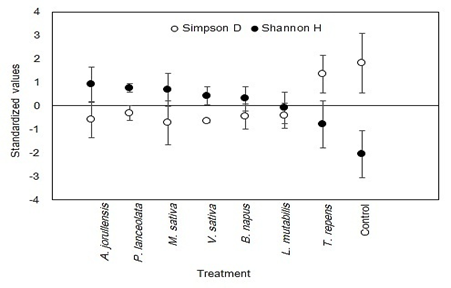
Fig. 6 Comparison of the mean values of diversity indices H 'and S in each treatment and each depth in the experimental plot El Fondo in Southwestern Colombia. The values are standardized to z units to facilitate their observation and comparison. Vertical bars represent 95 % confidence intervals.
TABLE 1 Abundance of invertebrate individuals by Family in each green fertilizer treatment per m2 at the experimental plot El Fondo in Southwestern Colombia
| Functional group | Families | Fertilizer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | V. sativa | P. lanceolata | M. sativa | T. repens | B. napus | L. mutabilis | A. jorullensis | Total | ||
| Depredate | Acaridae | 0 | 112 | 64 | 0 | 16 | 48 | 64 | 32 | 336 |
| Carabidae | 16 | 0 | 64 | 112 | 0 | 48 | 0 | 576 | 816 | |
| Coccinellidae | 48 | 0 | 96 | 48 | 48 | 0 | 16 | 16 | 272 | |
| Cryptopidae | 0 | 32 | 64 | 0 | 0 | 48 | 0 | 16 | 160 | |
| Elateridae | 0 | 16 | 0 | 0 | 0 | 32 | 0 | 0 | 48 | |
| Hemerobidae | 0 | 0 | 32 | 48 | 16 | 32 | 16 | 32 | 176 | |
| Lycosidae | 16 | 16 | 0 | 0 | 0 | 16 | 0 | 32 | 80 | |
| Nabidae | 0 | 0 | 16 | 0 | 0 | 0 | 16 | 32 | 64 | |
| Vespidae | 0 | 32 | 0 | 0 | 16 | 16 | 0 | 0 | 64 | |
| Detritivores | Blattidae | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 16 |
| Entomobryidae | 0 | 32 | 16 | 0 | 0 | 0 | 0 | 0 | 48 | |
| Forficulidae | 0 | 0 | 16 | 96 | 16 | 0 | 0 | 0 | 128 | |
| Gelechiidae | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Miridae | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 16 | |
| Polydesmidae | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Porcellionidae | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Scarabaeidae | 48 | 208 | 128 | 144 | 32 | 320 | 224 | 80 | 1 184 | |
| Staphylinidae | 0 | 0 | 0 | 32 | 16 | 0 | 48 | 96 | 192 | |
| Collector | Elmidae | 0 | 0 | 16 | 16 | 16 | 0 | 0 | 48 | 96 |
| Stratyomidae | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 192 | 208 | |
| Tipulidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 32 | |
| Herbivores | Acridoidea | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 32 |
| Chrysomelidae | 0 | 0 | 0 | 80 | 16 | 0 | 0 | 0 | 96 | |
| Cicadidae | 0 | 0 | 0 | 0 | 16 | 16 | 32 | 0 | 64 | |
| Curculionidae | 0 | 0 | 32 | 80 | 0 | 128 | 16 | 80 | 336 | |
| Noctuidae | 0 | 0 | 0 | 0 | 16 | 0 | 32 | 32 | 80 | |
| Nymphalidae | 0 | 32 | 0 | 16 | 16 | 0 | 0 | 32 | 96 | |
| Pentatomidae | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Ptilodactylidae | 48 | 0 | 64 | 0 | 0 | 0 | 0 | 80 | 192 | |
| Soil engineers | Formicidae | 0 | 0 | 176 | 368 | 48 | 16 | 288 | 0 | 896 |
| Lumbricidae | 320 | 160 | 640 | 480 | 432 | 464 | 320 | 640 | 3 456 | |
| Drosophilidae | 0 | 0 | 0 | 0 | 0 | 144 | 0 | 16 | 160 | |
| Sp. 1 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 16 | |
| Sp. 2 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 16 | |
| Sp. 3 | 0 | 0 | 48 | 0 | 0 | 0 | 0 | 0 | 48 | |
| Total | 512 | 688 | 1 504 | 1 552 | 720 | 1 344 | 1 088 | 2 080 | 9 488 | |
Discussion
Among the diversity of soil invertebrates that were found in this experiment, we identified different functional groups associated with particular green fertilizers. The detritivores represented by the families of the millipedes (Polydesmidae), the woodlice (Porcellionidae) and some beetles (Scarabaeidae), were associated to the P. lanceolata, M. sativa, and B. napus. This association may be due to the habitat provided by these fertilizers, with high humidity, temperature, and the ease of the plant material to be degraded and consumed. The organisms belonging to these families feed mainly on decomposing detritus, releasing nutrients and contributing to increase their availability in the soil. This helps plant growth and the storage of carbon in the soil (Mohammad, Rajasekar, & Patil, 2017). Additionally, these organisms are sensitive to abrupt changes in humidity and temperature, so they tend to quickly disappear under stress conditions (Zerbino, 2005). This makes this group highly relevant as an ecological indicator of soil quality under agroforestry management.
A high abundance of individuals from Lumbricidae and Formicidae was associated with the A. jorullensis, V. sativa and B. napus fertilizers. These organisms belong to the functional group of soil engineers, linked to the improvement of the physical properties that favor the formation of aggregates, the retention of water, the gaseous exchange and the mineralization of organic matter (Lavelle, 2000; Cabrera, Robaina, & Ponce De León, 2001; Cunha et al., 2016). The presence of these families has repercussions on the infiltration processes in the soil, since their organisms form channels that allow the movement of water. They also help the root development for different plant species (Domínguez, Aira, & Gómez, 2009) and serve as indicators for the biological quality of soils (Cairo, 2003).
The functional group of the predators, mainly represented by earwigs and some beetles from Carabidae and Coccinellidae, indicate complex trophic networks in the invertebrate community (Gullan & Cranston, 2000; Rousseau, Fonte, Téllez, Van Der, & Lavelle, 2013). The high abundance of these organisms associated with the A. jorullensis and P. lanceolata treatments indicate predation functions, particularly important over those pest species usually harmful for the crops (Funichello, Costa, & Gil, 2012). In Nariño, potato crops the most important for the economy of the Andean region- are attacked by aphids (virus vectors), who affect the seed production. However, this attack is significantly reduced by Coccinellidae organisms, which act as biological controllers («FEDEPAPA», sf).
A. jorullensis is known for having leaves with C/N rates around 20.6, lightly lignified and nitrogen rich (Mora, 2006), which is of great benefit for the soils due to rapid decomposition and higher N availability for decomposer organisms (Wagner & Wolf, 1998). Furthermore, A. jorullensis leaves also provide higher moisture to the soil, favoring the occurrence of organisms that decompose organic matter (Cabezas, Peña, Díaz, & Moreno, 2008). B. napus leaves has been found to favor the phosphorous and nitrogen re-cycling, modifying the composition of soil invertebrate communities (Arévalo, 2000).
In addition, due to climatic conditions, Genoy soils maintain a relatively high humidity throughout the year, a factor that guarantees a mitigating effect on temperature changes to achieve the colonization of fauna (Lavelle & Spain, 2001).
The higher individual’s abundance and familie’s richness of soil invertebrates found in depth 0-10 cm and is mainly explained by the reduction of the organic matter content and the oxygen at greater depths, which provide habitats and food for their development (Castro, Burbano, & Bonillam 2007). Our results agree with Delgado, Burbano and Silva (2010), who report the highest values in abundance and species richness in the surface soil layer (0-10 cm). Similarly, Villalobos et al. (2000) concluded that the abundance of soil invertebrates decreases with depth. However, another possible explanation is related to the time required for the fertilizer to reach deeper soil layers, which will additionally depend on porosity, hydraulic conductivity and rates of decomposition of organic matter. Most likely, the 45 days after incorporation in our experiment were not enough to trigger ecological changes below 20 cm.
For biomass, the results were inverse to the abundance and richness, since maximum values were found for Control, and minimum for A. jorullensis treatment. Because of the negative correlation between richness-biomass and abundance-biomass, the existence of an ecological trade-off between these variables seems to emerge. This would mean that, although green fertilizers have the capacity to promote the colonization of diverse invertebrates, the price is a decrease in the biomass. Since abundance increased with richness, it is itself not an explanation for the biomass. Our data do not allow us to properly explain this result; however, we hypothesize that it could be related to lower body sizes characteristic of those invertebrates that had a closer association for certain fertilizers. For example, in general, Tipulidae -unique under A. jorullensis fertilization and Stratyomidae shared only between A. jorullensis and L. mutabilis fertilizations have smaller mean body sizes (Hjorth-Andersen, 2015) than the invertebrates found in Control. Another hypothesis is higher competence intensity in treatments with more diversity, which implies that the available resources must be shared among more species and individuals. However, further research and data is needed to prove this hypothesis.
In general, these results allow us to highlight the big effects of the A. jorullensis and B. napus as green fertilizers in triggering the diversity of soil invertebrates and propose them as effective treatments to conserve soil functions in the region. Nevertheless, forthcoming studies should consider the importance of creating mixtures of different fertilizers to regulate/maintain functionality in the soil. It is also important that new studies identify specimens to the species level, since this could inform about more specific functional groups, host-fertilizer associations and the benefits for the soil.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

