Introduction
The genus Oecopetalum Greenm. & C.H. Thomps. (type species = O. mexicanum) was described from the state of Veracruz, Mexico, and assigned originally to the family Icacinaceae (Greenman & Thompson, 1914). Two more species were later added: O. greenmanii (Standley & Steyermark, 1940) and O. guatemalense (Howard, 1940). The genus can be recognized by the following combination of characters: tall trees with malpighiaceous hairs with unequal arms; inflorescence a large dichotomous cyme; flowers with an accrescent calyx and fleshy petals, with well-developed central nerves and margins on the inner faces; stamens with a large anther, connective in the form of an "X" in cross-section and introrse dehiscence. These characters are very distinctive among members of Icacinaceae s.l. (Howard, 1942; Kårehed, 2001). The genus is endemic to the New World, found in southern Mexico and Central America.
The first phylogenetic study of the Icacinaceae, based on combined molecular and morphological characters, showed Oecopetalum to be part of the ‘Emmotum group’ comprising six genera: Calatola, Emmotum, Oecopetalum, Ottoschulzia, Platea, and Poraqueiba (Kårehed, 2001). Two others studies (Angulo, Duno de Stefano, & Stull, 2013, Byng, Bernardini, Joseph, Chase, & Utteridge, 2014) suggested that Oecopetalum is the sister group of Ottoschulzia. Finally, a comprehensive phylogenetic analysis of the clade Lamiidae, using extensive data from the plastid genome, proposed a new classification of the whole clade, including the recognition of a reduced Icacinaceae (with 23 genera and 160 species) and an expanded Metteniusaceae (with 11 genera and 59 species; Stull, Duno de Stefano, Soltis, & Soltis, 2015). Although Metteniusaceae was previously considered monotypic, this expanded circumscription included Emmotum and related genera from the New and Old Worlds. Notably, Oecopetalum was found to be sister to the Asian monotypic genus Pittosporopsis, which previously had never been included in a phylogenetic analysis (Stull et al., 2015). This relationship had never been suggested before in the literature.
There is only one systematic treatment of Oecopetalum, including fewer than ten herbarium specimens (Howard, 1940). It was mentioned that the recognized species are similar but distinguishable from each other based on the number of secondary nerves in the leaves, the size of the flowers, and the general leaf shape. However, these differences might not be sufficient for the recognition of three separate species. In this study, therefore, we evaluate the hypothesis that the genus Oecopetalum is monophyletic and includes three species (Howard, 1940). It is possible that the genus only comprises two species (Standley & Steyermark, 1949; Gutiérrez-Báez, 1994). We also evaluate which genus is the closest relative of Oecopetalum, given that recent studies have suggested two possible alternatives: Ottoschulzia (Angulo et al., 2013; Byng et al., 2014) and Pittosporopsis (Stull et al., 2015), although the first two studies did not include Pittosporopsis.
Our general objective is to assess the morphological variability of the genus based on examinations of herbarium material, and to evaluate the monophyly of the genus and confirm its sister group based on phylogenetic analyses using the plastid genes ndhF and matK.
Materials and Methods
The morphological variability of the genus was evaluated using herbarium material (A, CHAPA, CHIP, CICY, ENCA, ENCB, F, GH, HUH, HULE, K, MEXU, MO, NY, US, XAL, acronyms as in Thiers (2010). Character data were obtained from hydrated flowers or direct observation of herbarium material with a Nikon SMZ800 microscope. Terminology follows the Systematic Association Committee for descriptive biological terminology (1962) for leaf shape and apex.
DNA from fresh leaves or herbarium samples was obtained (Table 1) using the DNeasy Plant Mini Kit (QIAGEN Inc., Valencia, California) following manufacturer’s Protocol. The plastid genes matK and ndhF were used to facilitate compatibility with already-available datasets for Icacinaceae s.l. (e.g., Kårehed, 2001; Angulo et al., 2013; Byng et al., 2014); these genes have also proven useful for resolving genus-level relationships in Icacinaceae s.l. and angiosperms in general (e.g., Hilu et al., 2003; Angulo et al., 2013). To date, there have been no published studies using nuclear genes (other than 18S rDNA; Kårehed, 2001) for phylogenetic analyses of Icacinaceae or Metteniusaceae. Previously generated sequences of matK and ndhF (Kårehed, 2001; Angulo et al., 2013) were obtained from GenBank. Four new sequences were generated for this study. Table 1 shows accession numbers for all the sequences examined. PCR amplifications were performed using an Applied Biosytems Veriti 96 Well Thermal Cycler. Reagents volumes and PCR conditions were as follows:
matK: 25 µL of mix containing 7.1 µl H2O, 2.5 µl buffer, 2.5 µl MgCl2, 1 µl primer matK472F, 1 µl primer matK1248R, 5 µl Solution Q, 2.5 µl dNTPs, 0.4 µl TAQ polymerase, and 3 µl DNAc. PCRs were conducted using the following protocol: 94 ºC x 4 min + 35 cycles (94 ºC x 45 s + 48 °C x 48 s + 72 ºC x 60 s) + 72º x 5 min. The primer information for matK472F (5’-CCC RTY CAT CTG GAA ATC TTG GTT C-3’) and matK1248R (5’-GCT RTR ATA ATG AGA AAG ATT TCT GC-3’) was obtained from Yu, Xue and Zhou (2011).
ndhF: 25 µL of mix containing 10.8 µl H2O, 2.5 µl Buffer, 1 µl MgCl2 ,1 µl primer 972F, 1 µl primer 2110R, 5 µl Solution Q, 1.5 µl dNTPs, 0.2 µl TAQ polymerase, and 2 µl DNAc. PCR’s were conducted using the following protocol: 94 °C x 4 min + 35 cycles (94 °C x 45 s + 49 °C x 90 s, 72 °C x 3 min), 72 ºC x 7 min. The primers 972 F (5’-GTC TCA ATT GGG TTA TAT GAT G-3’), and 2110 R (5’-CCC CCT AYA TAT TTG ATA CCT TCT CC-3’) were used (Olmstead & Sweere, 1994).
TABLE 1: matK and ndhF sequences used in this study
| - | matK | ndhF |
| Calatola mollis Standl. | 1974ZAF2352347476C | 17E6ZAB609617476C |
| Calatola costaricensis Standl. | 18B2ZAB056071485C | 17E6ZAB610618484C |
| Calatola laevigata Standl. | 18B2ZAB046061490C | 18B2ZAB078083489C |
| Cassinopsis ilicifolia Sleumer | AJ429312.1 | AJ429110.1 |
| Emmotum nitens (Benth.) Miers | KT738339.1 | KT738623.1 |
| Icacina senegalensis A. Juss. | AJ429313.1 | AJ429111.1 |
| Mappia mexicana B.L. Rob. & Greenm. | KT738351.1 | KT738635.1 |
| Metteniusa tesmannianna (Sleumer) Sleumer | KT738354.1 | - |
| Oecopetalum greenmanii Standl. & Steyerm. | R. Duno de Stefano 2938 | R. Duno de Stefano 2938 |
| Oecopetalum greenmanii | R. Duno de Stefano 2938 | R. Duno de Stefano 2938 |
| Oecopetalum greenmannii | R. Duno de Stefano 2938b | - |
| Oecopetalum mexicanum Greenm & C.H. Thomps. | KT738359.1 | KT738642.1 |
| Ottoschulzia rhodoxylon (Urb.) Urb. | KT738361.1 | KT738644.1 |
| Pittosporopsis kerrii Craib | KX526705.1 | KT738646.1 |
| Platea latifolia Blumer | KR531369.1 | KT738647.1 |
PCR products were sent to Macrogen Corea (http://www.macrogen.com/) for sequencing. The resulting DNA sequences were assembled and edited using BioEdit v 7.0.9 (Hall, 1999). The edited sequences in PhyDE v.0.9971 (http://www.phyde.de/download.html) were compared to the genetic data on GENBANK using the BLASTN tool. Each gene region (including the new and previously generated sequences) was separately aligned using ClustalW (Thompson, Higgins, & Gibson, 1994). Afterwards, alignments were manually refined according to criteria published by Wheeler (1996) to optimize homology. JmodelTest (Posada, 2008) was used to assess the best evolutionary model for each alignment suggested by the Akaike Information Criterion (AIC); GTR + I + G was selected for both ndhF and matK.
An exploratory phylogenetic analyses were performed with NONA (Goloboff, 1999), which was spawn through Winclada (Nixon, 2002). To perform the parsimony analyses, uninformative characters were removed from the analysis and all statistics were calculated without them. To search for the shortest trees derived from this matrix, we employed the Parsimony Ratchet (Nixon, 1999), of which five hundred iterations under Fitch parsimony were run with the following search settings: 20 trees hold at each iteration, and 10 % of the characters sampled in each iteration. Consensus trees were calculated using the strict consensus option in the program. To compare the internal support of clades, a 1 000 iteration bootstrap analysis was performed with 1 000 search repetitions and one tree held per iteration.
Bayesian analysis was performed with MrBayes v.3.2.5 (Ronquist et al., 2012). We performed a partitioned analysis, using the models inferred above for the two individual gene regions. The analysis included the default parameters of the software and was run for 5 million generations, with sampling every 1 000 generations and 25 % ‘burnin’ for two independent runs. Convergence between the runs was confirmed with Tracer v1.6 (Rambaut & Drummond, 2007). Posterior probabilities (PP) of ≤ 0.95 were considered weakly supported whereas PPs of 0.95-1.0 were deemed strongly supported (Alfaro, Zoller, & Lutzoni, 2003). Missing data were coded as “?”.
Results
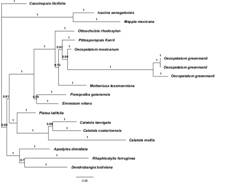
Phylogenetic analysis: The aligned matrix was 1 976 bp in length, 376 characters (19.03 %) were phylogenetically informative. The parsimony analysis yielded 16 equally most parsimonious trees of 694 steps, a consistency index (CI) of 71, and a retention index (RI) of 71. The strict consensus of the most parsimonious trees identifies Oecopetalum as a monophyletic with 88 % bootstrap, and Pittosporopsis as its sister genus with 72 % bootstrap. The topology of the strict consensus tree is similar to the Bayesian analysis, thus only the last one is included.
The Bayesian analysis showed a tree (Fig. 1) with a trichotomy formed by 1) Cassinopsis; 2) Icacina + Mappia (both clades form Icacinaceae s.s.; Stull et al., 2015), and 3) the ‘clade A’ that includes the taxa corresponding to Metteniusaceae sensu Stull et al. (2015). Clade A also includes a trichotomy formed by three clades: 1) Apodytes + Dendrobangia + Rhaphiostylis; 2) Calatola + Platea, and 3) the ‘clade B’ that includes Emmotum, Metteniusa, Oecopetalum, Ottoschulzia, Poraqueiba, and Pittosporopsis. In clade B, there are two subclades: Emmotum + Poraqueiba, and the ‘C clade’ containing Metteniusa, Oecopetalum, Ottoschulzia, and Pittosporopsis. In clade C, Ottoschulzia is sister to the rest of the taxa and Metteniusa is sister to Oecopetalum + Pittosporopsis. In general, the posterior probabilities values are low with the exception of Icacina + Mappia and the relationships of the three accessions of Oecopetalum greenmanii with posterior probabilities greater than 0.90. Pittosporopsis is the sister group to Oecopetalum with a posterior probability support value of 0.80.
Morphological examination: We studied 93 specimens of the genus Oecopetalum from numerous herbaria, providing a more complete survey of morphological variation than possible in previous work (Howard, 1942). The study of this material allowed us to recognize two patterns of morphological divergence associated with the size of the flowers and the number of secondary nerves, with both variables associated. In other words, one grouping of specimens had small flowers up to 5.5 mm long and a leaf blade with seven to twelve pairs of secondary nerves, whereas the other grouping of specimens had large flowers up to 9 mm long and a leaf blade with four to eight pairs of secondary nerves. The first morphological group corresponds to Oecopetalum greenmanii and the second to O. guatemalense and O. mexicanum. Finally, we did not observe morphological differences between the latter two taxa and consequently we consider them conspecific.
Taxonomic treatment: Oecopetalum Greenm. & C.H. Thomps., Ann. Miss. Bot. Gard. 1(4): 408. 1914. Type: Oecopetalum mexicanum Greenm. & C.H. Thomps.
The generic epithet comes from house (((((() and petal ((((((((), in reference to the space that exists on each side of the middle nerve of the petals and in which the stamens are positioned.
Trees up to 30 m tall. Young branches cylindrical, generally all parts glabrous or slightly sericeous-puberulent, becoming glabrous with time; trichomes simple, formed by malpighiaceous hairs, with unequal arms, falling early. Leaves simple, alternate, without stipules; petiole up to 2 cm long, weakly furrowed, without a clear abscission line at base, glabrous to sericeous-puberulent; blade subcoriaceous, turning brown when dry, generally oblong to narrowly oblong, rarely elliptic, or slightly obovate, glabrous or slightly sericeous-puberulent on the abaxial face, especially on the main nerve, becoming glabrous over time, glabrous on the adaxial side; apex acuminate or acute, rarely obtuse; margin entire; base attenuate to round; nerves penninerved, brochidodromous, main vein prominent on the abaxial side, slightly depressed and visible on the adaxial side, 4-10 (12) pairs of secondary nerves, conspicuous, alternating; tertiary nerves forming a conspicuous reticulum. Inflorescences up to 10 cm, axillary or falsely terminal, forming dichotomous cymes, densely flowered, axes and branches sericeous-puberulent, peduncle up to 3 cm long, green, white or reddish; bracts along the axes, concave, narrowly ovate to ovate, sericeous on the abaxial side, glabrous on the adaxial side, green, white or reddish; pedicels absent or up to 0.5 cm long, sericeous; bracteoles 1-2, concave, ovate to narrowly ovate, sericeous on the abaxial face, glabrous on the abaxial side, green, white or reddish, sometimes with simple protuberances. Flowers pentamerous, actinomorphic, hermaphroditic; one or two bracts per flower, at the base of the pedicel or in the middle, green, white or reddish, sometimes represented by small protuberances. Calyx slightly campanulate, persistent and accrescent in the fruit, sericeous in the abaxial face, lobes visible, triangular, concave; apex acute; margin entire, green. Corolla white, petals free, narrowly elliptical or narrowly oblong, sparsely sericeous or glabrous on the abaxial surface, hairy on the adaxial surface, mainly on the main nerve, slightly reflex; medium veins thickened and prominent in the adaxial surface; apex acute, slightly inflected; margin thickened towards the apex, green, white or reddish. Stamens alternate and coherent with petals, free; filaments thickened, straight, glabrous, white; anthers elongated, longer than the filament, sagittal, basifixed, slightly divergent at the base, yellow, theca separated by the connective, dehiscent by longitudinal grooves, introrse; connective narrowly ovate, "X" shaped in cross-section. Disk absent. Pistils conical, glabrous, rarely slightly hairy, ovary 1-locular, 2 seminal rudiments pendulous, collateral; style up to 0.4 mm long; stigma capitate or slightly bilobed. Fruit a subglobose drupe, slightly compressed giving rise to two small and almost inconspicuous ribs, glabrous; apex apiculate with persistent style of up to 0.5 cm long; the calyx persistent; triangular lobes, glabrous; exocarp reddish-brown, rugose; scarcely fleshy mesocarp; endocarp thin, hard; seed solitary.
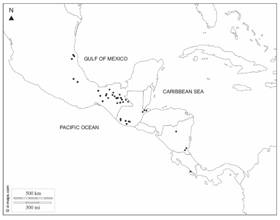
Distribution: Oecopetalum is endemic to the Neotropics, distributed in North America (Chiapas, Oaxaca, Tabasco, and Veracruz of Mexico) and Central America (Guatemala, Nicaragua, and Costa Rica). It has not been collected in El Salvador or Honduras. Together with Calatola, this genus represents the northernmost limit of Metteniusaceae in the Neotropics.
Uses and common names: Oecopetalum mexicanum and O. greenmanii have edible fruits. The first species is known in Veracruz as cachichín, which is a Totonaca word meaning “bitter fruit” (Lascurain-Rangel, Avendaño-Reyes, & López-Binnqüist, 2014); Oecopetalum greenmanii is known by a range of names; in Oaxaca, Chiapas, and Tabasco it is called kakatez (or kakaté) (Tzeltal language), cacaté, jacaté, jamajuquilla (Tojolobal language), cacaté and jamacuquiaca (Zoque language, in Atakapa) (Martínez, 1987).
Seeds of both species (cachichín and cacaté) have a bitter taste, the aroma and texture being similar to the peanut (Lascurain et al., 2013). It is consumed at any time of the day, usually toasted and sometimes boiled. Sale represents an infrequement, temporary income for many families and is part of the local culture (Lascurain, López, & Zamora, 2009; Lascurain, López-Binnqüist, & Emery, 2016). Seeds of O. mexicanum contains high levels of protein (19.37 %) and polyunsaturated fatty acids (60.02 %). The highest percentage (0.04 %) of sugars occurs when the seed is toasted, 0.02 % when it is boiled, and 0.03 % raw (Carballo, 1996). The nutrient values (proteins, fats and sugars) are lower than those reported for Arachis hypogaea (Hernández et al., 2013).
The seed collection of Oecopetalum mexicanum is carried out during April and May. The fruits are collected from the ground with the aid of a stick. Cachichín harvesting is a traditional event, a form of entertainment, and an opportunity to spend time with friends and family. The amount of fruit collected can vary from 7 to 30 kg per day. People who go in groups and collect for commercial purposes can collect up to 500 kg or more in 15 or 20 days during the harvesting season. When collectors come home with the load, the fruit is spread out on the floor in every room, even under the bed and furniture, and left to dry in the shade for about 20 days. The dried fruit can be stored for up to five months if kept dry in plastic bags.
The wood of cachichín is used for the construction of houses and for firewood. It has been reported that, in green condition, it has similar resistance to that of pine (Lascurain et al., 2007). In Chiapas the cacaté is used as a shade tree on the coffee farms, with the name of palo quemado or cacaté, and it is also used for firewood.
Key to the species of Oecopetalum
1. Flowers 5.0-5.5 mm long; corolla 5.0-6.0 mm long, white on the abaxial surface and reddish on the adaxial surface; stamens 4.5-5.0 mm long; pistil 1.3-1.5 mm long; leaf blade with 7-12 pairs of secondary nerves O. greenmanii
2. Flowers 7.0-9.0 mm long; corolla 7.5-8.3 mm long, white on both surfaces; stamens 5.0-7.0 mm long; pistil 2 mm long; leaf blade with 4-8 pairs of secondary nerves O. mexicanum
Oecopetalum greenmanii Standl. & Steyerm., Publ. Field Mus. Nat. Hist., Bot. Ser. 22 (3): 154. 1940. Type: Guatemala: Departament of Izabal, Río Dulce, 2-4 miles west of Livingston, on south side (left-hand side going up the rivers), at sea level, April 16, 1940, J. A. Steyermark 39526 (holotype, F). Fig. 2A, Fig. 2B, Fig. 2C, Fig. 2D, Fig. 2E, Fig. 3
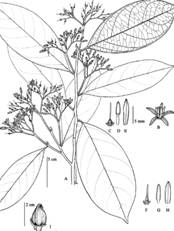
Fig. 2 Oecopetalum greenmanii A) Leaves, B) Inflorescence; C) Single fruit; D) Detail of flower; E) Fruits. (Photos: Dr. Gustavo A. Romero González).
Trees 5-30 m tall. Leaves 13-27 cm long; petiole 0.8-2 cm long, slightly crooked, glabrous to sericeous-puberulent; lamina subcoriaceous, obovate, oblong, narrowly oblong or elliptic, rarely ovate, 12-25 x 3.5-9 cm, glabrous or slightly sericeous-puberulent on the abaxial surface, becoming glabrous, glabrous on the adaxial surface; apex shortly acuminate or acute; margin entire; base attenuate; 7-12 pairs of secondary nerves visible. Inflorescences up to 10 cm long, axillary or falsely terminal, cyme with all parts sericeous-puberulent, peduncle up to 3 cm, sericeous-puberulent; bracts ovate, 2 x 1 mm, sericeous-puberulent on the abaxial side, glabrous on the adaxial side; bracteoles 1-2, ovate to narrowly ovate, 1 x 0.5 mm, sericeous-pubescent on the abaxial face, glabrous on the adaxial face, sometimes with simple protuberances. Calyx slightly campanulate, persistent and accrescent in the fruit, sericeous on the abaxial side; lobes triangular, 0.7-1 x 0.5-0.7 mm. Corolla white-light reddish, with narrowly oblong petals, 5-6 x 1.5-1.8 mm; scarcely sericeous or glabrous on the abaxial surface, slightly hairy on the adaxial surface on the main nerve; apices slightly reflexed. Stamens 4.5-5 mm long; filament 0.5-1.5 mm long; anthers 4 mm long. Pistil conical, 1.3-1.5 mm long, glabrous, rarely slightly hairy; style 3-4 mm long; stigma capitate or slightly bilobed. Fruit a subglobose drupe, slightly compressed on the longitudinal axis, giving rise to two ribs, 2-2.5 mm long, 1.5-1.8 mm wide, 1.8-2 mm in diameter, apex apiculate, with persistent style of up to 5 mm long.
Distribution and ecology: In Mexico (Chiapas and Tabasco), Guatemala (Izabal, San Marcos, Solola, and Suchitepequez), Nicaragua (Atlántico Sur, Jinotega, and Río San Juan) and Costa Rica (Puntarenas) (Fig. 3); in lowland forests to montane forests, 100-2 100 m.
Material examined: see Appendix 1
Taxonomic observations: The morphological differences with Oecopetalum mexicanum are primarily the size of the flower and especially the stamens, for which the ratio of anther to filament is greater in O. greenmanii. Also, the number of secondary nerves is useful for determination, but this character is not as clear as the size of the flowers. Another difference in the living plant is the color of the flower; O. greenmanii has white-light reddish petals, whereas the petals of O. mexicanum are strictly white. The fruit of O. greenmanii is also harder and slightly bigger.
Oecopetalum mexicanum Greenm. & C.H. Thomps., Ann. Missouri Bot. Garden 1: 408, plate 25. 1914. Type: México: Veracruz, Sierra Madre near Miscantla (sic), August, 1912, C. A. Purpus 6159 (holotype, F; isotype, MO). Fig. 3, Fig. 4A, Fig. 4B, Fig. 4C, Fig. 4D, Fig. 4E, Fig. 4F.
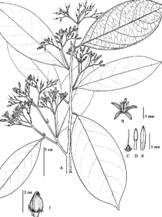
Fig. 4 Oecopetalum mexicanum A) Habit; B) Flower; C) Pistil detail without petals; D) Stamen; E) Petal, F) Single fruit. (O. mexicanum G. Ibarra et al. 3667, MEXU, Drawing Bruno Manara)
Oecopetalum guatemalense R.A. Howard, J. Arnold Arbor. 21: 483, tab. 3. 1940. Type: Guatemala: Suchitepequez: Finca Moca, 1 140 m., Jan. 8, 1935, Skutch 2028 (holotype, A; isotype, F, NY).
Trees 9-20 m tall. Leaves subcoriaceous; petiole 0.7-2.0 cm long, slightly crooked, glabrous, sparsely sericeous to sericeous-puberulent; oblong, narrowly oblong, narrowly ovate or elliptical, 9.0-30.0 x 3.5-10.0 cm, glabrous or sericeous-puberulent on the abaxial surface, especially on the main nerve, which becomes glabrous; apex short acuminate or acute, rarely obtuse; margin entire; base flattened to rounded; secondary veins 4-8 pairs, visible. Inflorescences up to 10 cm long, axillary or falsely terminal, top dichotomous, all parts sericeous-puberulent; peduncle up to 2.5 cm long, sericeous-puberulent; bracts narrowly ovate or ovate, 3.0 x 1-1.5 mm, sericeous-puberulent on the abaxial surface; bracteoles 1-2, ovate, 2.0 x 1.0 mm, sericeous-puberulent on the abaxial surface, sometimes with protuberances. Calyx slightly campanulate, persistent and accrescent on the fruit, sericeous on the abaxial surface, glabrous on the adaxial surface; lobes visible, triangular, 1 x 1 mm; apex acute. Corolla white, petals narrowly elliptical or narrowly oblong, 7.5-8.3 x 1.7-2.0 mm; scarcely sericeous or glabrous on the abaxial surface, glabrous on the adaxial surface, slightly reflexed. Stamens 5.0-7.0 mm long; filament 2.2-3.0 mm long; anthers 4.0-4.2(5.3) mm long. Pistil conical, 2.0 mm long, glabrous, rarely slightly hairy; style 4.0 mm long; stigma capitate or slightly bilobed. Fruit a subglobose drupe, slightly compressed on the longitudinal axis, giving rise to two ribs, 2.0-2.5 long, 1.5-1.8 mm wide,1.8- 2.0 mm diameter, apex apiculate; the persistent calyx 1.0-1.2 cm long, lobes triangular, 0.5 x 0.5 cm, glabrous.
Distribution and ecology: Southern Mexico (Chiapas, Oaxaca, Tabasco, and Veracruz) and Guatemala (Quetzaltenago, San Marcos, and Suchitepéquez). Montane forests, between 800 and 2 000 m.
Specimen examined: See Appendix 1.
Taxonomic discussion: O. guatemalense is considered conspecific with O. mexicanum as we did not find any significant morphological differences between these taxa. Our results support the findings of several previous papers (Standley & Steyermark, 1949; Gutiérrez-Báez, 1994) and call for the recognition of a single species: O. mexicanum (incl. O. guatemalense).
Discussion
Oecopetalum is monophyletic and diagnosable by a unique suite of morphological characters; it also shares multiple morphological features with Pittosporopsis, including a cymose inflorescence (short in Pittosporopis, longer in Oecopetalum), stamens as long as petals, and a short style persistent in fruit. Our phylogenetic analyses recovered Oecopetalum and Pittosporopsis as sister genera, albeit with only moderate to weak support (posterior probabilities 80); however, this relationship was strongly supported in the plastome analyses of Stull et al. (2015).
Regarding the New World genera, some flower and fruit characters of Ottoschulzia resemble Oecopetalum. There is great morphological homogeneity in the populations of Oecopetalum that occupy an approximate extension area of 287 500 km2 from Oaxaca and Veracruz in Mexico to Puntarenas in Costa Rica. The genus has not been recorded in El Salvador or Honduras.
Within Oecopetalum, there are a few conspicuous differences between the two recognized species: the size of the flowers and the ratio of anther to filament. Another difference is the color of the inflorescence and flower; in Oecopetalum greenmanii the peduncle and main branches of the inflorescence are white and reddish; the petals are white reddish on the abaxial surface and reddish on the adaxial surface. Oecopetalum mexicanum has greenish or white inflorescences and flowers.
There is not a general geographical pattern regarding the morphology. We believed that the movement of propagules by local people from one site to another has disrupted any geographical distinction (some herbarium specimens blur this geographic distinction), for example a Pacific Coast and Atlantic Coast pattern or a North and South pattern.
Recent work has made important systematic changes to the family Icacinaceae s.l., beginning with the initial segregation of its members into four families, Cardiopteridaceae, Icacinaceae, Pennantiaceae and Stemonuraceae (Kärehed, 2001), followed by a further reduction of Icacinaceae (to 23 genera) and the recognition of an expanded Metteniusaceae with 11 genera (Stull, Duno de Stefano, Soltis, & Soltis, 2015). Comparing the results of this paper to those of previous studies (e.g., Käreded, 2001; Lens et al., 2008; Byng et al., 2014; Stull et al., 2015) is challenging due to differences in sampling of both taxa and molecular markers. The present tree (Fig. 1) is intermediate between the results of Byng et al. (2014) and Stull et al. (2015). The first study recovered Ottoschulzia as sister to Oecopetalum (Byng et al., 2014), which is supported by their shared distribution (the New World) and by floral similarities, but Pittosporopsis was not included in this study. The results presented here (although weakly supported) show Pittosporopsis, a monotypic genus from Southeast Asia, as the sister group of Oecopetalum, corroborating the results of Stull et al. (2015). Despite the low support (p.p) for a sister relationship between Pittosporopsis and Ottoschulzia, previous plastome analyses (Stull et al., 2015) that had good taxon sampling, showed maximum support for such a relationship. Also, our exploratory parsimony analysis shows the same topology despite featuring low bootstrap support (72 %). This transatlantic relationship is neither new nor surprising within the families Icacinaceae and Metteniusaceae. In the first case, there is the sister group relationship of Mappia (America) and Nothapodytes (Asia). In the second case, there are several transatlantic sister groups: the genera Calatola (America) and Platea (Southeast Asia), and Apodytes (Africa), Dendrobangia (America), and Rhaphiostylis (Africa and Madagascar). Several possible geologic scenarios might explain this pattern. Early studies on plant geography often attributed transatlantic disjunctions to the fragmentation of Gondwana (e.g., Raven & Axelrod, 1974), but the timing of this geologic process is not concordant with the fossil record of most disjunct angiosperm groups (e.g., Manchester, 1999; Christenhusz & Chase, 2012), e.g., Icacinaceae (Stull, Herrera, Manchester, Jaramillo, & Tiffney, 2012; Allen, Stull, & Manchester, 2015; Del Río, Haevermans, & De Franceschi, 2017). Instead, the Boreotropical model (Wolfe, 1975), in which high-latitude land bridges facilitated the spread of tropical taxa during the globally warm early Paleogene, better matches available phylogenetic and fossil evidence available for Icacinaceae, Metteniusaceae, and other plant groups, such as legumes (Lavin & Luckwoc, 1993) and Malpighiaceae (Davis, Bell, Fritsch, Mathews, & Donoghue, 2002).
The genus Oeceopetalum is monophyletic and includes two morphologically similar species, differing primarily in terms of floral features. Both species can be regarded as good candidates for future silvicultural programs. Pittosporopsis, an Asiatic genus, is the sister group of Oecopetalum. This transatlantic relationship is recurrent in Icacinaceae and Metteniusaceae. The Boreotropical model (high-latitude land bridges facilitated the spread of tropical taxa during the globally warm early Paleogene) appears to be the best explanatory model for this disjunction in light of available phylogenetic and fossil evidence.












 uBio
uBio