Introduction
Biological communities are experiencing rapid changes in Neotropical ecosystems driven by species local extinction and colonization of new sites, resulting by the interaction and additive effects of factors such as climate change, emerging diseases and habitat degradation; yet there is emerging evidence of adaptive potential of species to respond to environmental change (Mendelson et al., 2004; Colwell, Brehm, Cardelús, Gilman, & Longino, 2008; Bickford, Howard, Ng, & Sheridan, 2010; Ryan et al., 2014; Acosta-Chaves & Cossel, 2016; Lister & García, 2018; Voyles et al., 2018). The way in which biological communities change over time due to these and other anthropogenic and natural pressures has become a relevant issue in community ecology (Ryan et al., 2014; Lister & García, 2018; Voyles et al., 2018). Costa Rican amphibians provide an interesting model to study these patterns due to the population decline that several species had suffered during the last decades ( Pounds & Crump, 1994; Lips, 1998, Lips et al., 2006; Whitfield et al., 2007; Ryan et al., 2014; Acosta-Chaves, Bolaños, Spínola, & Chaves, 2016). Climate change (Pounds, Fodgen, & Campbell, 1999) and emergent diseases (Lips et al. 2006) have been pointed out as the main causes of these declines. The consequences seems more obvious at intermediate elevations where many species of the families Bufonidae, Ranidae, Hylidae, Strabomantidae and Craugastoridae rapidly declined (Lips, 1998; Lips et al., 2006), after being historically common and abundant (Scott, 1976). In the lowlands and middle elevations more gradual declines also resulting in extirpation throughout this same time period has been reported mainly in forest leaf-litter anurans (Whitfield et al., 2007; Hilje & Aide, 2012, Ryan et al., 2014; Ryan et al., 2015; Acosta-Chaves et al., 2016).
Despite this negative scenario for amphibian communities, some hope still remains. Recently, numerous frog species previously considered vulnerable, critical endangered or extinct have been rediscovered in distinct localities of Costa Rica (Abarca, Chaves, García-Rodríguez, & Vargas, 2009; Hertz et al., 2012; Castro-Cruz & García-Fernández, 2012; González-Maya et al., 2013; Chaves et al., 2014; Kubiki, 2016; Jiménez & Alvarado, 2017). Also, during the enzootic phase of Bd-fungus in central Panama, some species have gradually recover numbers (Voyles et al. 2018). On the other hand, some litter species survive in altered areas, even with similar or higher success than in pristine forests (Hilje & Aide, 2012; Acosta-Chaves, Chaves, Abarca, García-Rodríguez, & Bolaños, 2015; Acosta-Chaves et al., 2016). However, those scenarios varies at local scale (Abarca-Alvarado, 2012; Acosta-Chaves et al., 2015), and there are probably individual responses at the species and population level depending on factors such as elevation and type of forest (Ryan et al., 2014). Thus, biological monitoring across sites in different life zones is necessary to help understand this process and inform conservation decisions at local and regional scales.
Here, we present the case of several species occurring at premontane forests in the western portion of Cordillera Volcánica Central in Costa Rica, specifically in the Alberto Manuel Brenes Biological Reserve (known as Reserva de San Ramón, hereafter RSR). This is a protected area where substantial herpetological research has been conducted since the 1970s. Until the mid 1990s a total of 30 amphibians were recorded by Bolaños & Ehmcke (1996), but after a sampling effort between 2012 and 2015, six new species were added to the previous amphibian checklist (Morera-Chacón & Sánchez-Porras, 2015). More recently the Red-eyed Tree Frog (Agalychnis callydrias) was reported in the site (Morera-Chacón & Jiménez-Castro, 2017). These authors also mentioned some apparent changes in species richness; however, they lacked the data that could help understand those population oscillations. Along with the changes in richness they mentioned, we also have detected changes in species abundance through the years based on personal observations and data collected. Thus, this study aims to 1) provide evidence of changes in the amphibian richness of RSR across a ~30 year period, and 2) quantify changes in the assemblage, and relative abundance, of the leaf-litter frog community in the premontane forest of San Lorencito River area. For this, we compared our diversity data with a difference of ~18 years between sampling periods.
Materials and methods
Study site: The RSR ranges from 550 to 1 650 m a.s.l., comprising transition from tropical forest to premontane forest, premontane forest and lower montane forest (7 800 Ha) (Morera-Chacón & Sánchez-Porras, 2015). The RSR has a larger area in the Cantón of San Ramón (Alajuela Province) and a smaller portion that belongs to the Cantón of Montes de Oro (Puntarenas Province), Costa Rica. The RSR has 95 % of its area in the Caribbean basin (Brenes, 2007). We conducted this study nearby the San Lorencito River Station “Rodolfo Ortiz Vargas” of RSR (hereafter SLRS), located in the premontane forest belt that belongs to Los Ángeles de San Ramón District (10°13’42.245” N & 84°38’30.556” W; datum: WGS84). The average temperature is 21 °C, the relative humidity about 98 % and the average precipitation is 3 460 mm; more historical, climatic, geographical and biological information about RSR can be found in Rodríguez-Salazar (2000) (Fig. 1 and Fig. 2).
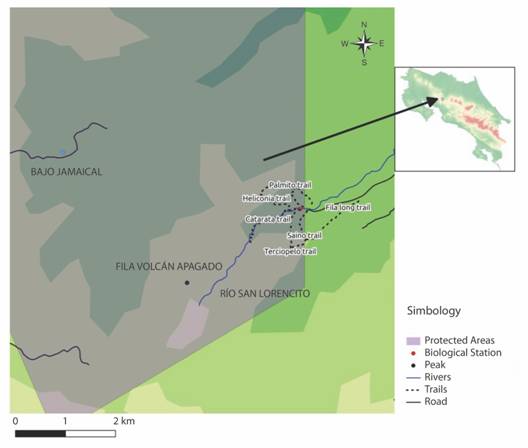
Fig. 1 Trail system of Reserva San Ramón, including our surveyed trails: Sendero Catarata, Sendero Terciopelo, Sendero Heliconia and Sendero Saíno in San Lorencito River Station.

Fig. 2 Riparian forest in Reserva San Ramón (Sendero Catarata trail) (A). Common leaf-litter anurans in the Reserva San Ramón: Pristimantis cruentus (B, C), Craugastor fitzingeri (D), Craugastor crassidigitus (E), Craugastor cf. stejnegerianus (F), Craugastor bransfordii (G), Pristimantis ridens (H) and Lithobates warszewitschii (I). Photos by Víctor Acosta-Chaves.
Diversity data collection: Our data derives from a series of non-continuous standardized surveys, but also includes museum data and field notes. Primarily, we analyzed 19 standardized nocturnal surveys from January 1994 to June 1995 (one survey per month), and nine nocturnal surveys dispersed from November 2011 to October 2012 (one survey per month). Those standardized surveys were conducted along the San Lorencito River and the north slope of the Volcán Apagado area (between 900 to 1 400 m a.s.l.) (Catarata, Heliconia, Terciopelo and Saíno trails) (Fig. 1 and Fig. 2).
We surveyed transects of 200 x 2 m in each trail by sampling session (2-3 persons), between 20:00 to 22:00 during 1994-95 and 2011-12. Sampling implies active searches for amphibians on different substrates such as leaf-litter, understory vegetation, fallen logs, rocks, both in the trail and the river to identify individuals to species level (Crump & Scott, 1994; Mendelson et al., 2004; Morera-Chacón & Sánchez-Porras, 2015). Additionally, we used field notes, specimens deposited in the collection of Museo de Zoología of Universidad de Costa Rica and recent literature to complement richness data since the mid 1980s to 2010s.
Data analysis: General species richness. To evaluate the level of similarity of amphibian richness from 1980s to 2010s in the SLRS area and surroundings, we ran a cluster analysis (Jaccard similarity index, paired group) with the software PAST 3.2 (Hammer, Harper & Ryan, 2001) to compare between richness data from 1980s, 1990s and 2010s.
Leaf-litter frog community. We limited this analysis to anurans from the families Craugastoridae, Strabomantidae and Ranidae that inhabit the leaf-litter (Table 1). To compare similarity in the species richness, between 1980s, 1990s and 2010s, we ran a cluster analysis (Jaccard similarity index, paired group linkage) with the software PAST 3.2 (Hammer, Harper, & Ryan, 2001).
TABLE 1 Amphibians reported for the San Ramón Reserve. Species detected in San Lorencito River Station area and surroundings (SLRS) at different time periods, and records of species from other zones (OZ) in the reserve, are classified here
| Taxa | SLRS | OZ | ||
| 1980s | 1990s | 2010’s | ||
| Caudata | ||||
| Plethodontidae | ||||
| Bolitoglossa alvaradoi | X | |||
| Nototriton gamezi | X | |||
| Anura | ||||
| Bufonidae | ||||
| Atelopus varius | X | |||
| Incilius coniferus | X | X | ||
| Incilius melanochlorus | X | X | ||
| Rhinella horribilis | X | |||
| Centrolenidae | ||||
| Cochranella granulosa | X | X | ||
| Espadarana prosoblepon | X | X | ||
| Hyalinobatrachium vireovittatum | X | |||
| Sachatamia ilex | X | X | ||
| Craugastoridae | ||||
| Craugastor andi* | X | |||
| Craugastor bransfordii* | X | X | X | |
| Craugastor crassidigitus* | X | X | ||
| Craugastor fitzingeri* | X | X | X | |
| Craugastor fleischmanni | X | |||
| Craugastor megacephalus* | X | |||
| Craugastor melanostictus | X | |||
| Craugastor podiciferus | X | |||
| Craugastor ranoides | X | |||
| Craugastor cf stejnegerianus* | X | X | X | |
| Strabomantidae | ||||
| Pristimantis altae | X | |||
| Pristimantis caryophyllaceus* | X | X | ||
| Pristimantis cerasinus | X | X | ||
| Pristimantis cruentus* | X | X | X | |
| Pristimantis ridens* | X | X | X | |
| Eleutherodactylidae | ||||
| Diasporus diastema | X | X | X | |
| Diasporus hylaeformis | X | X | ||
| Hylidae | ||||
| Tripion spinosus | X | |||
| Duellmanohyla rufioculis | X | X | ||
| Ecnomiohyla miliaria | X | |||
| Ecnomiohyla sukia | X | |||
| Scinax elaeochroa | X | |||
| Smilisca phaeota | X | X | X | |
| Smilisca sordida | X | X | ||
| Leptodactylidae | ||||
| Leptodatylus savagei | X | |||
| Phyllomedusinae | ||||
| Agalychnis callydrias | X | |||
| Agalychnis lemur | X | |||
| Ranidae | ||||
| Lithobates taylori | X | |||
| Lithobates vaillanti | X | |||
| Lithobates vibicarius | X | |||
| Lithobates warszewitschii* | X | X | ||
(*) species included in the leaf-litter frog composition analysis.
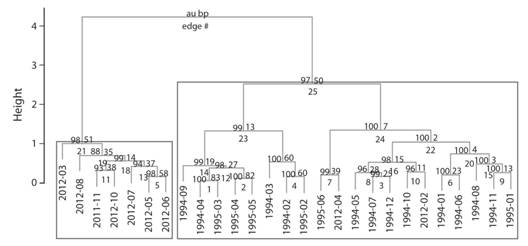
To compare dissimilarity in diversity between nocturnal standardized surveys from 1994-1995 and 2011-2012, and cluster similar surveys by month, we ran a cluster analysis (with Bray-Curtis dissimilarity index, Ward linkage) using the package PVCLUST (Suzuki & Shimodaira, 2006) in R (Ihaka & Gentleman, 1996). PVCLUST allows bunch data after calculates the probability value for each cluster with resampling techniques, providing the approximately unbiased P- value (AU) and the bootstrap probability (BP) (Suzuki & Shimodaira, 2006). Finally, we estimated the percent of relative abundance for those leaf-litter anurans during each sampling period, and compared their frequencies with a Chi square test.
Results
General species richness. We recorded a total of 41 amphibians for the RSR (Table 1, Appendix), but just 36 species were historically detected in the premontane belt of SLRS and surroundings (Table 1). Our effort added Bolitoglossa alvaradoi, Craugastor melanostictus, C. fleischmanni, C. podiciferus and Ecnomiohyla sukia to the previews known checklist of RSR (Morera-Chacón & Sánchez-Porras, 2015). We also changed the species named as Eleutherodactylus sp1 and Eleutherodactylus sp2 by Bolaños and Ehmcke (1996), and treat them here as Craugastor cf. stejnegerianus and Pristimantis cruentus, respectively (Table 1, Appendix).
The similarity of species richness in the premontane elevation is ~40 % when we compare data from the 1980s against data from 1990s and 2010s (Fig. 3A). In addition, there is a slightly higher similarity in richness between 1980s and 2010’s than between 1980s and 1990s at premontane elevation (Fig. 3A).
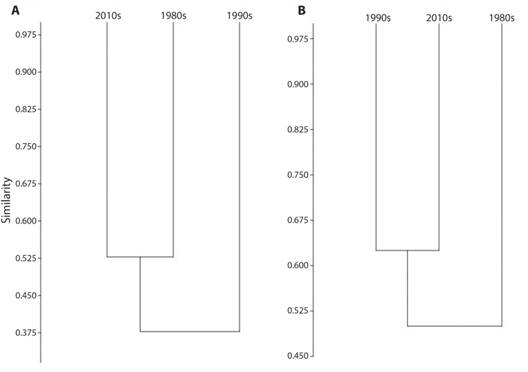
Fig. 3 A. Cluster analysis of amphibian richness (Jaccard index, paired groups) of San Lorencito River Station at different periods. B. Cluster analysis of leaf-litter anuran richness (Jaccard index, paired groups) of San Lorencito River Station at different periods.
Leaf-litter frog community. We found a total of 13 leaf-litter anurans that were present in the SLRS area before the enigmatic decline period. During mid 1990s, only five species were recorded; however at least nine of the species detected before the decline were found in the 2010s (Table 1, Fig. 2). Five species of leaf-litter anurans have never been re-detected during 1990s and 2010s surveys (Table 1, Fig. 4). Finally, Pristimantis altae, Craugastor crassidigitus and Lithobates warszewitschii were not detected during the standardized surveys of the 1990s, but were sporadically seen during that time in the studied area (Bolaños, pers. obs.) (Table 1, Fig. 4). The richness of leaf-litter frogs is ~ 50 % similar between the 1980s and 1990s-2010s, while the richness between 1990s and 2010s was ~ 65 % similar (Table 1, Fig. 3B).
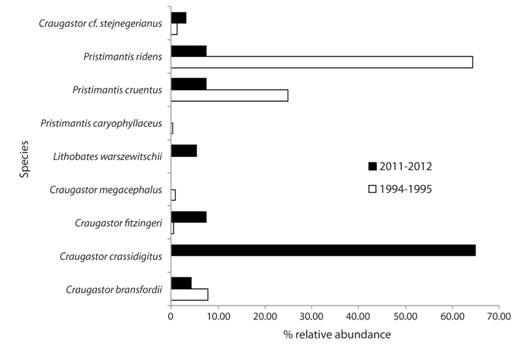
Fig. 4 Percent of relative abundance of leaf-litter frogs detected during standardized surveys in San Lorencito River Station at different times.
Our community dissimilarity dendrogram shows distinct differences between diversity surveys from both periods (1994-1995 and 2011-2012); those surveys were clearly separated between decades (Fig. 5). We found differences in the relative abundance of the compared leaf-litter species (X2 = 7036.45; d.f. = 17; P < 0.05). Currently, C. crassidigitus is the most common amphibian although it was almost absent during the 1990s (Fig. 4). While some species disappeared from recent surveys, other anurans increased their abundance during 2010s, especially C. fitzingeri and L. warszewitschii (Fig. 4). In contrast, Pristimantis ridens and P. cruentus, were the most frequent species during the mid 1990s, but showed an apparent decline, reaching similar abundances to uncommon species during that same time period such as C. fitzingeri (Fig. 4).
Discussion
Before discussing about the changes in species richness occurred in San Ramón Reserve, we want to clarify the inclusion or omission of some species in our list, to avoid confusion with previous published efforts. Morera-Chacón and Sánchez-Porras (2015), for example, identified as C. bransfordii some specimens stated as Eleutherodactylus sp1 in Bolaños and Ehmcke (1996). Here we report those specimens as C. cf. stejnegerianus based on clear morphological differences with C. bransfordii, and similarities that resembles to C. stejnegerianus clade (Chaves, pers. obs.). Bolaños & Ehmcke (1996) reported another unidentified species as Eleutherodactylus sp2, we here included those specimens under P. cruentus. The alpha taxonomy of the genus Pristimantis, -including the clade of P. cruentus-, is difficult and present a high degree of cryptic diversity ( Rivera-Correa, Jiménez-Rivillas, & Daza, 2017), then, we adopted a conservative position due to most Costa Rican frogs assigned to P. cruentus require further study. Finally, the original Ecnomiohyla miliaria listed by Bolaños and Ehmcke (1996) was indeed an E. sukia according to Savage and Kubicki (2010). Nevertheless, there is a specimen of E. miliaria (UCR 5142) from Finca Orlich (Alajuela, Costa Rica; circa 800 masl) (Savage & Kubicki, 2010), a site in the proximities of RSR. We included both species because the two types of Ecnomiohyla could coexist in the extensive area that belongs to RSR. The addition of new species to the general checklist, from different sites than SLRS, reveals that some unexplored areas from the RSR require further survey to complement the known amphibian richness for this protected area.
Our results suggest that the species richness of SLRS have drastically changed after the mid 1990s. The differences recorded in the amphibian assemblage across decades in RSR could be caused by a combination of population declines, disease dynamics, detection issues and ecological interactions. For example, elusive canopy and fossorial species (e.g. salamanders and canopy tree frogs) have always been considered rare, and could be perfectly not detected under long sampling periods due to their own ecology (Savage, 2002). But, when species that were historically common neither are not recorded during multiple sampling efforts across circa 30 years, we are likely witnessing a rapid extirpation from the locality. Two evident examples of such circumstances are Craugastor ranoides and Atelopus varius, once among the most common species in Costa Rican rainforests (Savage, 2002; Puschendorf, Chaves, Crawford, & Brooks, 2005; González-Maya et al., 2013). C. ranoides gradually disappeared from most of the country with the exception of the driest forest of Guanacaste, where the elevated temperature mitigates the effect of the Bd-fungus (Puschendorf et al., 2005). A similar situation experienced A. varius in Costa Rica where is currently restricted to some localities under 1000 masl in the foodhills of Central and South Pacific slope (González-Maya et al., 2013). Other frogs such as Craugastor andi and Pristimantis altae apparently disappeared from SLRS area, or their populations suffered an extreme decrease on density, reason why were not recorded by recent efforts in the last ten years.
In addition to issues related to species detection or rapid disappearances (Ryan et al., 2014); our analysis identified a radical change in the diversity of leaf-litter frogs in SLRS. We focused our discussion on what happened to species that are still often observed, or have become persistent, after the enigmatic decline period because our data identified population oscillations for most of those species in our study site. Some historically common species declined significantly, while several previously rare or presumed extirpated species became fairly common in San Ramón Reserve, which is unusual because terrestrial frogs are not expected to have extreme population fluctuations (Ryan et al., 2014). A similar phenomenon has been reported in the Caribbean lowlands; where several leaf-litter frogs are still absent in several localities where they were once common (Hilje & Aide, 2012). Also, it is known that population density varies across sites and/or time for several species of direct development leaf-litter frogs occurring in La Selva (Whitfield et al., 2007), Las Cruces (San Vito, Costa Rica) and Rincón de Osa (Osa Peninsula, Costa Rica) (Ryan et al., 2014; Ryan et al., 2015). Some hypotheses for these declines include the increase in mean temperature and rainfall and decrease in leaf-litter depth, which could affect amphibians negatively (Whitfield et al., 2007; Ryan et al., 2014; Ryan et al., 2015).
Our study site shares some direct development leaf-litter frog species with La Selva, Las Cruces and Rincón de Osa, and several species at those sites are also facing population changes across time. For example, C. megacephalus, C. bransfordii, P. ridens and P. cruentus suffered a gradual decline in the lowlands of La Selva and surroundings (Whitfield et al., 2007; Hilje & Aide, 2012), while P. ridens and P. cruentus declined in the premontane forest of Las Cruces (Ryan et al., 2014; Ryan et al., 2015). Contrary to C. bransfordii, C. cf. stejnegerianus seems relatively stable in San Ramón as well as in Las Cruces (Ryan et al., 2014; Ryan et al., 2015). C. bransfordii have experienced historical declines in density after the dry season of 1994 in SLRS, as the declines mentioned in the lower montane forest of Río Macho (Cartago, Costa Rica) for C. underwoodi, its taxonomic and ecological equivalent species (Acosta-Chaves et al., 2016). The lower densities reported for those species during 2011-2012 in comparison with 1994-95 in SLRS could be driven by La Niña, as was observed in Las Cruces (Ryan et al., 2014; Ryan et al., 2015) and Río Macho (Acosta-Chaves et al., 2016).
In the case of C. crassidigitus we found an inverse pattern to the observed by Ryan et al. (2014; Ryan et al., 2015). This species declined significantly in the lowlands of Rincón de Osa and is barely common in Las Cruces; however in SLRS it became the most common species even though it was rarely seen during the mid 1990s and early 2000s. In the foothills of Alto de Campana (Panama), C. crassidigitus was the least affected species after Bd-fungus was found in 2006 (Woodhams et al., 2008), and its populations were abundant and stable along premontane forest of La Amistad International Park (Costa Rica-Panamá) where other species declined (Alvarado, 2012). Finally, L. warszewitschii is a common premontane amphibians in Costa Rica nowadays, and its populations possibly are increasing (Alvarado, 2012; Leenders, 2016). The population oscillation of P. ridens and L. warszewitschii in Panama (Voyles et al., 2018) coincides with our findings in SLRS.
The dynamic of Bd-fungus in RSR have not been evaluated, however, our changes in similarity of amphibian richness of SLRS through decades could be explained by a similar pattern to the documented in central Panama by Voyles et al. (2018): after an epizootic phase of Bd-fungus emergence (for SLRS mid 1990s), the host amphibian community become more similar between the enzootic phase (for SLRS 2010s) and pre-disease phase (for SLRS 1980s). In contrast, this pattern does not fit as well when we compared only the species that inhabit the leaf- litter layer. Their model based on host responses against Bd-fungus would explain the population oscillations in our study site for anurans such as P. ridens, P. cruentus, C. fitzingeri, C. crassidigitus and L. warszewitschii; however, some of our species in SLRS (especially harlequin toads and several direct development leaf-litter frogs) did not survived and subsequently recovered in our site. Other leaf-litter species such as C. bransfordii are declining in lowlands where the Bd-fungus should not be lethal, mainly for habitat changes due to climate change (Whitfield et al. 2007). Those different situations support the hypothesis of individualized responses to environmental changes by species depending on the site conditions and on elevation (Ryan et al., 2014; Ryan et al., 2015).
Variation in the composition of a biological community, driven by the extinction, decline or colonization of species, can be the result of direct or indirect competition (e.g. apparent competition) between a species with skills to adapt or invade a new ecosystem, or to use a pathogen as an advantage upon competitors (Price, Westoby, & Rice, 1988; Bickford et al., 2010). Recently, in Panama, was reported that C. crassidigitus produces skin secretions with and inhibitory effect against Bd-fungus (Voyles et al., 2018). If Bd-fungus were the main cause of declines in RSR, currently dominant species such as C. crassidigitus or L. warszewitschii could have developed host resistance against the pathogen during the enzootic phase of the illness in our premontane site. Further research should focus on why frogs such as the cited before become dominant at intermediate elevation after a population crisis, because Bd-fungus was more virulent for amphibian communities there (Puschendorf, Bolaños, & Chaves, 2006) than in the lowlands (e.g. Rincón de Osa) (Puschendorf et al., 2005; Ryan et al., 2014; Ryan et al., 2015), and those species could success after an apparent competition with other previously common anurans (Price et al., 1988). Determining how and why some anurans survived, and even flourished, could be critical for the in-situ and ex-situ conservation strategies for other common and endangered species coexisting in tropical forests.
Finally, climate change is a factor that will gradually influences the composition of amphibian communities of Costa Rican premontane forest in a near future. Changes in composition of communities of small mammals, reptiles, amphibians, plants and insects from temperate and tropical zones (including Costa Rica) have either been documented or are expected due to global warming and subsequent ecological interactions (Moritz et al., 2008; Colwell et al., 2008; Bickford et al., 2010; Acosta-Chaves & Cossel, 2016; Lister & García, 2018). Being conscious that the diversity of amphibians is shifting through relative short periods of time in the Neotropic because the synergy of multiple factors, we suggest continue the biological monitoring in San Ramón and other areas from Costa Rica during the upcoming years. Even if the current scenario for many unique amphibians from the premontane tropical forest is unclear, is crucial to understand how this ecosystem gradually resists and adapts to this catastrophic time of biodiversity loss.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio