Chemical defense refers to any mechanism by which plants or animals utilize a chemical substance to avoid being predated or stop an attack that has already began (Wilsdon, 2009). These chemical substances can either be synthetized by the organism from its own genome and metabolism, or be obtained from an external source. Examples of chemical defense can be found on many animal species. A lot of cases have been reported for both vertebrate and invertebrate fauna, the latter presenting most of the occurrences. In vertebrates, chemical defense has mostly been reported for reptiles and amphibians. Other groups, like mammals and birds, contain just a few known cases (Savitzky et al., 2012). It wasn’t until relatively recently that the first case was reported for a bird species. Dumbacher, Beehler, Spande, Garraffo, and Daly (1992) confirmed Pitohui, an endemic genus to New Guinea, to be the first avian group to present some sort of toxin (batrachotoxin, in this case). Since then, several more avian species have been reported to present chemical defense, including: Ifrita kowaldi, Plectropterus gambesis, Ergaticus ruber, Bonasa umbellus, Phaps elegans, and Phaps chalcoptera (Bartram & Boland, 2001).
Pheucticus chrysopeplus, the species evaluated, belongs to the family Cardinalidae. They possess a solid yellow color in head and chest, black wings and a lightly white spotted black tail. It has a distinctive massive black beak (Fig. 1). They inhabit Mexico and Guatemala and tend to inhabit clear and dry forests from 300 to 1 850 m above sea level (Fagan & Komar, 2016). This species is listed as endangered by the Guatemalan List of Endangered Species (CONAP, 2009). The assumption of its possible toxicity lies on its aposematic-like coloration. Aposematism is a mechanism by which animals advert their predators through a warn signal (vivid coloration or patterns) that they are toxic or distasteful (Weldon, 2000; Skelhorn & Rowe, 2007). P. chrysopeplus bright yellow and black plumage ties in with this definition. Another apparent indicator that drives us to test the possible toxicity of feathers of this species is the observation that local people avoid consuming this species due to it supposedly having unpleasant taste if it’s not adequately prepared and cooked, based on observations by Ariano-Sánchez & Salazar (2004-2017). In order to evaluate the toxicity of P. chrysopeplus, we realized alcoholic feather extracts and assessed their toxicity with a lethality test using Artemia salina as a model

Fig. 1 Images for (A) I. pustulatus (Common name: Streak-backed oriole), (B) P. chrysopeplus (Common name: Yellow grosbeak) and (C) I. pectoralis (Common name: Spot-breasted oriole). Source, in order of listing: Lee’s Birdwatching adventures plus, by Amy McAndrews; Aves de Costa Rica, by Jorge Chinchilla; Turismo de observación, by Raúl Vega.
Materials and methods
Study site: The study was carried out on Reserva Natural Privada para la Conservación del Heloderma (RNH), a protected area managed by local NGO Zootropic. It is located on El Arenal village, Cabañas municipality, Zacapa department, Guatemala (14°53’ N - 89°47’ W). Average annual precipitation is 815 mm, average temperature is 26.9 °C, average relative humidity is 71 % and annual evapotranspiration is 1 798.8 mm. Elevation ranges from 310-950 masl. Topography is mostly undulant, with many conglomerated steep cliffs. There’s presence of two main seasons, rainy season (June-October) and dry season (November-May). Landscape is composed by many patches of seasonally dry tropical forest and tropical thorn shrub, with a matrix of cornfields alongside some cliffs (Ariano-Sánchez & Salazar, 2015).
Plant species include Plumeria rubra (Apocynaceae); Bursera simaruba (Burseraceae); Nopalea guatemalensis, Opuntia decumbens, Pilocereus leucocephalus, Stenocereus pruinosus, Stenocereus eichlami (Cactaceae); Acacia picachensis, Leucaena collinsii, Lysiloma divaricatum, Mimosa platycarpa (Mimosaceae); Swietenia humilis (Meliaceae); Ximenia americana (Olacaceae); Bonellia macrocarpa (Theophrastaceae); and Karwinskia calderonii (Rhamnaceae). Based on its Importance Index, the dominant species are: Oak (Bucida macrostachya, Combretaceae), Quebracho (Lysiloma divaricatum, Mimosaceae), Yaje (Leucaena collinsii, Mimosaceae), Campón (Gyrocarpus americanus, Hernandiaceae), Organ cactus (Stenocereus pruinosus, Cactaceae) and Cabro’s fruit (Karwinskia calderonii, Rhamnaceae) (Ariano-Sánchez & Salazar, 2015).
Feather collection: We placed mist nets (6-8) on forest openings with high bird traffic on RNH. Nets were open from 06:00 to 10:00 and from 16:00 to 18:00 hrs. Nets were checked every 20 minutes for trapped birds. Feathers from P. chrysopeplus were collected per recommendations of the Protocol of Feather Sampling (UCLA, 2015). Feathers were used because, being the first line of defense against predators, they’re a logical functional repository for defensive toxic substances (Wieldon, 2000). Furthermore, Dumbacher et al. (1992) determined feathers contained the second highest concentration of toxins from several tissues of Pitohui sp., only below skin concentrations. Approximately 0.5 g of chest, head and dorsal feathers were collected, along with two tail feathers. To pluck feathers, they’re hold from their base and firmly pulled outward. Feathers were stored in Ziploc® bags separated by individual, and kept at 4 °C. Each bag was identified with individual identification number, date of sampling, and time. Every caught organism was marked with nail polish (to avoid recapture) and then freed.
Bioethics: Bird handling was made according to FAO’s Bird Handling and Ringing Techniques FAO (2007). All procedures here described were evaluated and approved by CEUCA-UVG (Committee of Ethics, Use, and Animal Care of Universidad del Valle de Guatemala).
P. chrysopeplus feather extract preparation: Feathers were cut and ground to small portions. Approximately 0.1 g of feather macerate was packed in a 0.5 mL microcentrifuge tube. Using a small needle, a tiny hole was drilled at the base of the tube. Feathers were soaked in 2 mL of ethanol per gram of feather and allowed to sit for 5 min, to allow toxins to dissolve in the ethanol. The 0.5 mL drilled tube was then placed inside a 2 mL microcentrifuge tube and centrifugated at 13 200 rpm during 5.5 min to filter the ethanol. The process was repeated three times, and the ethanol washes were combined and evaporated on a Shake n’ Bake at 60 °C. The resultant residue was resuspended on 2 mL of methanol per gram of feather. Extracts were stored at 4 °C following Dumbacher, Menon, and Daly (2009) methods.
Lethality test: Brine shrimps (Artemia salina) were used as a model to test feather extract toxicity. According to Wu (2014), its use is compatible with methanol as a solvent as they are very resistant to it, which is relevant for obtaining results without confusing effects of the solvent used. Sealed 1 000 mL micropipette tips were used as vials to serve at least 10 A. salina in 500 µL of a combination of artificial seawater (38 g sea salt/L deionized water), PBS buffer (pH 8), methanol, and feather extract, according to each case. Specific concentrations were used as described on Table 1.
Table 1 Composition of each treatment solution evaluated
| Treatment | Artificial seawater | - | Other substances |
|---|---|---|---|
| Control | 250 µL | + | 250 µL PBS buffer |
| Methanol | 495 µL | + | 5 µL methanol |
| Feather extract | 495 µL | + | 5 µL P. chrysopeplus feather extract |
Control treatment used PBS buffer as it prevents pH variability in the control group that may affect normal mortality of A. salina (Hamidi, Jovanova, & Kadifkova, 2014). Methanol treatment was used to assess the mortality of A. salina exposed to methanol used as solvent for the feather extracts produced. Lastly, the feather extract treatment was used to determine mortality of A. salina exposed to the extract, therefore determining any toxicity it may present. Feather extracts were evaluated at 1 % concentration under recommendations of Geethaa, Jayanthi, Poh, and Ming (2013). They described interference of common solvents used in brine shrimp lethality tests, including methanol, and suggest 1.25 % as the maximum concentration when evaluating toxicity of a substance that contains methanol as a solvent. Mortality was evaluated as the proportion of brine shrimp dead in each vial after 24 hours of exposure to each treatment.
We compared the mean mortality of each treatment (n = 50) through Kruskal-Wallis nonparametric tests, as the distribution presented by the data did not fit a normal distribution. Data distribution was evaluated through a ShapiroWilk test and histogram visual analysis. All analyses were made using JMP 5.0 software.
Results
Three P. chrysopeplus were captured and feathers were obtained as described in methods. As shown in Fig. 2, P. chrysopeplus feather extract produced a higher mortality percentage (29.3 ± 0.19) than compared to control (6.7 ± 0.11) and methanol (6.5 ± 0.09).
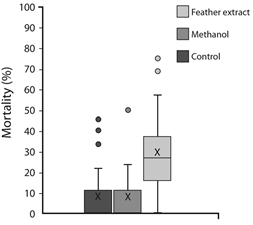
Fig. 2 Box plots of Artemia salina mortality after 24-hour exposure to each treatment (control, methanol, and Pheucticus chrysopeplus feather extract).
We found a significant difference in mortality between treatments (X2 = 65.25, P < 0.0001, n = 50). This finding makes P. chrysopeplus the first known toxic avian species of Guatemala.
Discussion
Brine shrimp lethality test allowed us to determine P. chrysopeplus feather extract produces higher mortality in A. salina than control and methanol treatments. This higher mortality is evidence of some sort of toxicity inherent to P. chrysopeplus feathers. We identify Pheucticus chrysopeplus as the first toxic bird described for Guatemala and Central America. This ties in to what’s known for the species, as it is not sought for consumption by local people. According to locals interviewed, it has an unpleasant taste. Feathers, therefore, may contain bioactive concentrations of one or various toxic substances. Toxicity may be an adaptive way to reduce predation in a harsh environment such as the dry forest, as even human populations are discouraged to hunt them.
This species plumage pattern is also very similar to other sympatric birds such as Icterus pectoralis and I. pustulatus (Aves: Icteridae), two poorly related bird species with which it cohabits in the seasonally dry forests of Guatemala. These similitudes might reflect mimicry existing between both groups, with one or more parties developing similar characteristics to others in order to reduce predation without actually possessing some sort of chemical defense (Audesik, Audesirk, & Byers, 2003). A similar case was reported for Pitohui dichrous and P. kirhocephalus where the plumage pattern of both species was similar in areas where they coexisted (Dumbacher & Fleischer, 2001).
P. chrysopeplus bright yellow and black plumage may be considered aposematic, considering this species toxicity (Weldon, 2000; Skelhorn & Rowe, 2007). Toxicity of P. chrysopeplus also evidences interesting links with I. pectoralis and I. pustulatus. These species plumages are very similar, even when they don’t belong to the same family, which might hint to a case of Batesian mimicry. Usually, bird’s plumage tends to be more alike the more related one species is with another. This is not the case for orioles, though. Species poorly related can share very similar plumage patterns. This is possible because plumage pattern can rapidly evolve as it is a sexually selected characteristic (Omland & Lanyon, 2000; Price, Friedman, & Omland, 2007). The resemblance of both oriole species to P. chrysopeplus may imply there is an ecological benefit in resembling its plumage pattern. In this case, P. chrysopeplus toxicity might indirectly benefit species that look similar to it, as they too will be avoided by predators under the presumption they are toxic as well (Audesirk et al., 2003). Another option, however, is that one or both oriole species possess some toxicity themselves. In this case, Mullerian mimicry might be in play.
With our findings, we raise awareness on the relevance of identifying the toxic substances present in P. chrysopeplus feathers, as they may provide some relevant medical or agricultural applications. Many toxins have been used for clinical and agricultural purposes, and there may be a use for these toxins as well (Gopalakrishnakone, 2015). This may also provide conservation incentives toward P. chrysopeplus, an endangered and rare species in Guatemala. Nevertheless, we acknowledge the results in this study are limited by the low number of individuals of P. chrysopeplus collected (n = 3). Toxic effects of feather extracts can only be awarded to at least a part of P. chrysopeplus population, until future studies confirm the same properties for a larger part of it. Likewise, the lack of a chemical characterization prevents us from giving a solid conclusion to the acquisition mechanism this species uses to generate or acquire said toxic substance. In light of this, we strongly suggest the continuation of the studying of this species. We also recommend that both oriole species (I. pectoralis and I. pustulatus) are evaluated in regard to toxicity of their feathers to have a better understanding of the ecological function of the resemblance in plumage pattern with P. chrysopelus.












 uBio
uBio 

