Garcinia hombroniana Pierre (Clusiaceae) is a tropical tree native to Malaysia, Thailand, Borneo and also found in the islands of Andaman and Nicobar (Abraham et al., 2008; Nazre, 2010). The root and leaves of G. hombronianaare generally used to treat itchiness and as a preventive medicine after childbirth (Nazre, Clyde, & Latiff, 2007). With its well developed root system and fast growth in natural environment, G. hombroniana is also used as rootstocks in improving fruiting quality of Garcinia mangostana Linn. and other species of Garcinia (Yaacob & Tindall, 1995).
Garcinia hombroniana is a seasonal fruit and propagated by seed. However, seeds are recalcitrant and their life span is short. Recalcitrant seeds usually undergo little or no maturation drying and they contain high moisture content after shedding (Noor, 2008). The seeds cannot be stored in conventional seed banks because they quickly lose viability and do not survive low moisture content (Rao, 2004). Cryopreservation is thus the most promising option for the long-term storage of recalcitrant seeded species (Walters, Berjak, Pammenter, Kennedy, & Raven, 2013).
Vitrification is the most commonly used technique in cryopreservation. This is a simple and reliable technique which allows much more selection of plant materials to be used for cryopreservation (Sakai & Engelmann, 2007). For this technique there are no special equipment needed and the procedure is fast as concentrated solutions are used which shorten the time and thus leading to fast recovery (Reed, 2008). This technique has been successfully studied for many plant species, including those of recalcitrant species, for example, Trichilia emetica Vahl. (Varghese, Berjak, & Pammenter, 2009), Nephelium ramboutan-ake (Labill.) Leenh. (Chua & Normah, 2011) and Parkia speciosa Hassk.(Nadarajan et al., 2008). However, this technique uses highly concentrated vitrification solution, which could cause injury by chemical toxicity or excess osmotic stress (Sakai, Hirai, & Niino, 2008). Hence, it is important to obtain the best solution and dehydration procedures to overcome this limitation.
Studies on cryopreservation of G. hombroniana have not been reported thus far. Since this species’ seed is relatively large in size and the availability of seeds depends on the fruiting season, in vitro seed cultures were used for shoot proliferation and shoot tips from these cultures were used as the starting materials for cryopreservation. Hence, the present study aims to investigate the response and tolerance of G. hombroniana shoot tips to cryopreservation using vitrification technique. Suitable preculture duration, loading solution, dehydration period and unloading solution were studied in order to obtain successful cryopreservation of this species.
Materials and methods
Plant materials: Ripe fruits of G. hombroniana were collected from the Garcinia germplasm collection at the Universiti Kebangsaan Malaysia, Selangor. Matured seeds with testa removed were sterilized (Ibrahim & Normah, 2013) and cultured on MS (Murashige & Skoog, 1962) medium with 2 mg/L BAP (benzylaminopurine), 0.3 M sucrose and 7 g/L Bacto agar to induce multiple shoot formation (Figure 1). The cultures were incubated under fluorescent light at 25 ± 2 ºC under a 16 h light/8 h dark photoperiod, with a light intensity of 22.26 µE cm-2 s-1 (3000 lux) and maintained by subculturing monthly. Shoot tips for the cryopreservation experiments were excised (1-2 mm long with two to three leaf primordia) from the shoots formed and cultured on MS medium for one week before use.
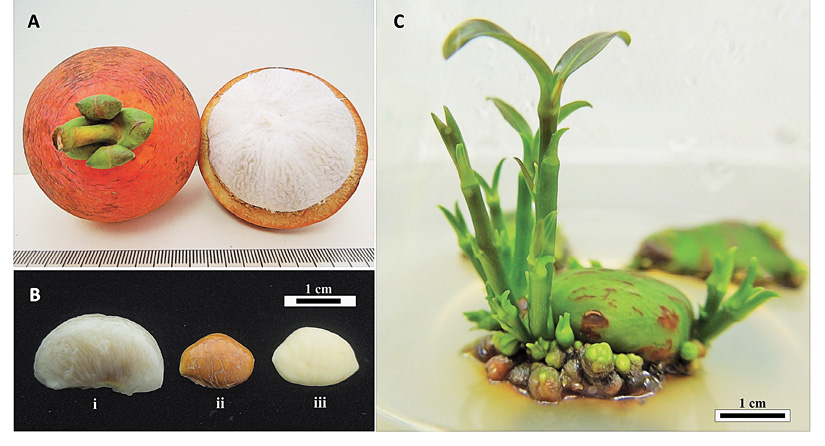
Figure 1 Garcinia hombroniana A. Fruit. B. Seed with aril (i) Seed with testa (ii) Seed without testa (iii). C. Multiple shoots formed from seed cultured in vitro.
Cryopreservation procedures: The vitrification protocol used in this study was based on Sakai, Kobayashi and Oiyama (1990). Excised shoot tips were precultured on solid MS medium containing 0.3 M sucrose for 48 h, loaded with 2 M glycerol and 0.4 M sucrose in liquid MS medium for 20 min, then dehydrated in PVS2 [30 % glycerol, 15 % ethylene glycol, 15 % dimethyl sulfoxide (DMSO) and 0.4 M sucrose in liquid MS medium] at 0 °C. The explants were then added with 0.5 mL fresh PVS2 before directly plunging them into liquid nitrogen (LN) for at least 1 h. Thawing was carried out rapidly in a water bath at 38 ± 2 °C for 1-2 min and later replaced with 0.4 M sucrose in MS solution for 20 min. Cryopreserved shoot tips were then transferred to sterilize filter paper over MS medium containing 2 mg/L BAP (plating). After 48 h, shoot tips were transferred to a recovery medium (MS supplemented with 2 mg/L BAP and 7 g/L Bacto agar). All solutions were prepared in liquid MS medium with pH at 5.8 and filter sterilized before use.
Preliminary test on PVS2 exposure: The response of shoot tips to PVS2 exposure was tested. After preculturing on MS medium with 0.3 M sucrose for 48 h, the shoot tips were exposed to 100 % PVS2 for 5, 10, 15, 20, 25, 30, 35, 40 and 45 min at 0 oC before being directly cultured on recovery medium.
Effects of PVS2 exposure durations on shoot tip survival: Shoot tips were exposed to 100 % PVS2 for 20, 25 and 30 min at 0 °C. All the excision, preculture, loading, LN exposure, thawing, unloading and recovery were carried out as mentioned in the cryopreservation procedure above.
Efficiency of stepwise PVS2 exposure to shoot tip survival: After preculturing and loading, shoot tips were exposed to PVS2 by stepwise approach. Three stepwise treatments which were T1 (25 min with 100 % PVS2), T2 (15 min with 50 % PVS2 followed by 10 min with 100 % PVS2) and T3 (10 min with 50 % PVS2 followed by 15 min with 100 % PVS2) at 0 °C. Excision, preculture, loading, LN exposure, thawing, unloading and recovery steps were carried out as mentioned in the cryopreservation procedure above.
Effect of different preculture durations on shoot tip survival: Preculturing was carried out on solid MS medium containing 0.3 M sucrose for 24 and 48 h, followed by loading and dehydration with PVS2 (T2-15 min in 50 % PVS2; 10 min with 100 % PVS2) exposure. Excision, LN exposure, thawing, unloading and recovery steps were as mentioned in the cryopreservation procedure above.
Effects of different loading solutions on shoot tip survival: After preculturing with 0.3 M sucrose for 48 h, three types of loading solution (0.4 M sucrose; 0.4 M sucrose + 1 M glycerol; and 0.4 M sucrose + 2 M glycerol) were tested for 20 min. Excision, preculture, dehydration with PVS2, LN exposure, thawing, unloading and recovery steps were carried out as mentioned in the cryopreservation procedure above.
Effects of different unloading solutions on shoot tip survival: After cooling and thawing steps, shoot tips were exposed to two unloading treatments with different sucrose concentration (0.4 M and 1.2 M sucrose) for 20 min. Excision, preculture, loading, dehydration with PVS2, LN exposure, thawing and recovery were carried out as mentioned in the cryopreservation procedure above.
Recovery assessment: The survival was assessed on the number of shoot tips that appeared green and expanded after treatments. Cultures were kept at 25 ± 2 ºC under a 16 h light/8 h dark photoperiod.
Water content determination: Immediately after each step (preculture, loading and dehydration with PVS2), shoot tips (ten for each replicate of three replicates) were weighed using four-decimal place analytical balance and dried in an oven at 103 oC for 16 h and after that 45 min in a desiccator. The shoot tips were then re-weighed to determine the dry weight. Water content was expressed on a dry-weight basis (g H2O per gram dry weight [g g-1]).
For all experiments, three replicates of ten shoot tips per replicate were used for each treatment. Data on survivals were collected every two/three days and shoot tips that remained green and expanded after cryopreservation were considered to have survived. Analysis of variance (ANOVA) was carried out and Duncan’s Multiple Range Test was used to compare the means. The statistical software SAS 9.3 (Statistical Analysis Systems) was employed.
Results
Preliminary test on PVS2 exposure: In this experiment, precultured shoot tips were dehydrated with 100 % PVS2 at 0 °C without LN exposure to test the ability of shoot tips to withstand PVS2 for extended periods of time. The result showed that the shoot tip survival decreased with the increase (from 5 to 45 min) in the duration of dehydration (Figure 2). Dehydration for 5 min gave the highest survival of shoot tips (100 %), and the survival decreased significantly from 10 and 15 min to 50 % for shoot tips dehydrated for 20, 25 and 30 min. With further dehydration of 35, 40 and 45 min the survival decreased further to 30 % (Figure 2). Dehydration duration of 20, 25 and 30 min were chosen for the following experiment.
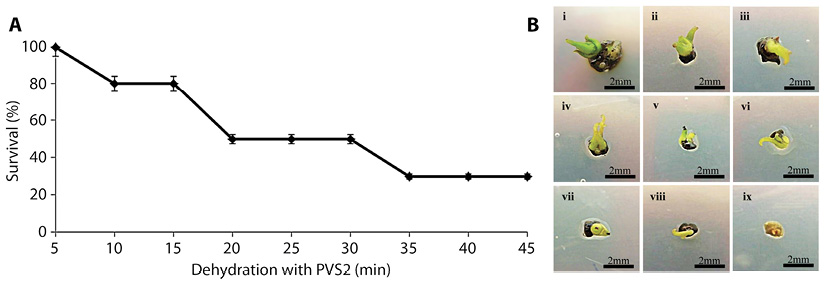
Figure 2 A. Percentage survival of G. hombroniana shoot tips after exposure to PVS2 solution for various durations. Bars represent standard deviation. B. Shoot tips that were dehydrated for different durations in PVS2 solution after one week on recovery medium. (i) 5 min (ii) 10 min (iii) 15 min (iv) 20 min (v) 25 min (vi) 30 min (vii) 35 min (viii) 40 min (ix) 45 min.
Effects of PVS2 exposure durations on shoot tip survival: Survival of shoot tips after dehydration with PVS2 for different durations is shown in Table 1. High survival (79.17 % to 91.67 %) was obtained for shoot tips not LN-exposed and the highest survival was for those without dehydration with PVS2 (91.67 %). However, for those LN-exposed, the survival was from 0 % to 20.83 %. There was no survival of LN-exposed shoot tips without PVS2 dehydration. The highest survival after exposure to LN (20.83 %) was obtained when the shoot tips were dehydrated with PVS2 for 25 min, though it is not significantly different from 20 and 30 min treatments. Dehydration with PVS2 for 25 min was chosen for the subsequent experiment.
Table 1: Effects of PVS2 exposure durations on survival of G. hombroniana shoot tips after five days
| on recovery médium | Survival (%) | |||
| 0 min | 20 min | 25 min | 30 min | |
| +LN | 0 | 12.50c | 20.83cb | 4.17c |
| -LN | 91.67a | 79.17a | 83.33a | 87.50a |
Means with the same letter are not significantly different at P ≤ 0.05 as determined by Duncan’s Multiple Range Test.
Efficiency of stepwise PVS2 exposure to shoot tip survival: Control shoot tips (not treated with PVS2) showed no survival after exposure to LN (Table 2). Stepwise dehydration procedures gave a higher recovery over that of one-step procedure. Treatment with 50 % PVS2 for 15 min followed by 100 % for another 10 min gave a slightly higher percentage of survival (41.67 %) than treatment with 50 % PVS2 for 10 min followed by 100 % PVS2 for 15 min, which gave 33.33 % survival. Those exposed directly to 100 % PVS2 for 20 min gave 25 % survival after LN exposure. Both stepwise procedures showed no significant difference from the one-step procedure after LN exposure. Nevertheless, since the shoot tips treated with 50 % PVS2 for 15 min followed by 100 % for another 10 min (T2) gave highest survival after LN exposure, this procedure was used for the subsequent experiments.
Table 2: Efficiency of stepwise PVS2 exposure on survival of G. hombroniana shoot tips after five days on recovery medium
| Survival (%) | ||||
| Control | T1 | T2 | T3 | |
| +LN | 0.00c | 25.00b | 41.67b | 33.33b |
| -LN | 91.67a | 70.83a | 75.00a | 66.67a |
Control - Without PVS2 exposure; T1 - 25 min with 100 % PVS2; T2 - 15 min with 50 % PVS2 followed by 10 min with 100 % PVS2; T3 - 10 min with 50 % PVS2 followed by 15 min with 100 % PVS2. Means with the same letter are not significantly different at P ≤ 0.05 as determined by Duncan’s Multiple Range Test.
Effect of different preculture durations on shoot tip survival: Shoot tips precultured for 48 h on MS medium with 0.3 M sucrose and exposed to LN gave the highest survival (75 %) after 4 days on recovery medium (Table 3). However, 50 % survival was obtained for those precultured for 24 h. The result showed significant differences in term of survival between the two treatments both with and without LN exposure. Preculture treatment for 48 h was chosen for subsequent experiment as this treatment gave the significantly highest survival after LN exposure.
Table 3: Effects of preculture duration on survival of G. hombroniana shoot tips after four days on recovery medium
| Survival (%) | ||
| 24 h | 48 h | |
| +LN | 50.00c | 75.00b |
| -LN | 75.00b | 95.83a |
Means with the same letter are not significantly different at p≤0.05 as determined by Duncan’s Multiple Range Test.
Effects of different types of loading treatment on shoot tip survival: The survival of shoot tips not LN-exposed ranged from 75 % to 95.83 %, and the survival was the highest when shoot tips were treated with 0.4 M sucrose (95.83 %). However, for those LN-exposed, treatment with 0.4 M sucrose gave significantly lower survival (8.33 %) compared to those with the addition of 1 M (45.83 %) and 2 M glycerol (33.33 %) (Table 4). Though there was no significant difference, loading treatment with the addition of 1 M glycerol was chosen for subsequent experiment as this treatment showed higher survival compared to treatment with 2 M glycerol.
Table 4: Effects of different loading solutions on survival of G. hombroniana shoot tips after four days on recovery medium
| Survival (%) | |||
| 0.4 M Sucrose | 0.4 M sucrose + 1 M glycerol | 0.4 M sucrose + 2 M glycerol | |
| +LN | 8.33d | 45.83c | 33.33c |
| -LN | 95.83a | 79.17ab | 75.00b |
Means with the same letter are not significantly different at P ≤ 0.05 as determined by Duncan’s Multiple Range Test.
Effects of different unloading solutions on shoot tip survival: In this experiment, the results showed that there were significant differences in terms of percentage survival for the cryopreserved shoot tips treated with the two unloading solutions (Table 5). Treatment with 0.4 M sucrose gave the highest survival of 66.67 %, while treatment with 1.2 M sucrose gave only 25.00 % survival. For shoot tips not exposed to LN, there was no significant difference between the two treatments with survival of 66.67 % and 62.50 % (Table 5).
Table 5: Effects of different unloading solutions on survival of G. hombroniana shoot tips after four days on recovery medium
| Survival (%) | ||
| 0.4 M sucrose | 1.2 M sucrose | |
| +LN | 66.67a | 25.00b |
| -LN | 66.67a | 62.50a |
Means with the same letter are not significantly different at P ≤ 0.05 as determined by Duncan’s Multiple Range Test.
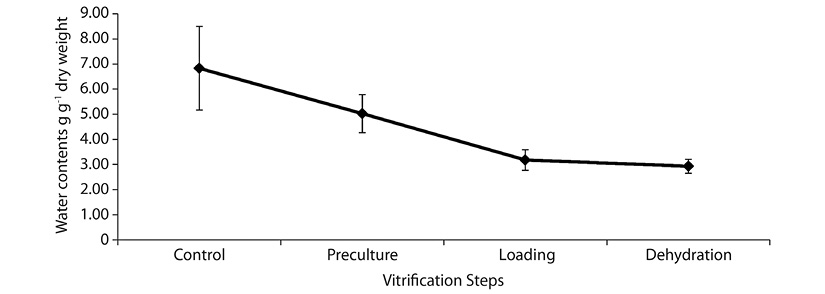
Determination of water content: The initial water content of freshly excised shoot tips (control) was 6.83 ± 1.66 g g-1 dw (dry weight). After preculture, the water content gradually reduced to 5.03 ± 0.76 g g-1 dw (Figure 3). The shoot tips continued to lose water during loading and desiccation steps to 3.18 ± 0.41 g g-1 dw and 2.93 ± 0.28 g g-1 dw respectively (Figure 3).
Discussion
Tolerance of shoot tips to different dehydration periods with PVS2 showed that 25 min was the best dehydration duration for G. hombroniana shoot tips. A stepwise protocol was also tested and gave a much better survival (41.67 %). PVS2 is known to be toxic to plant tissues (Volk & Walters, 2006). This solution dehydrates cells osmotically at nonfreezing temperature; hence, cells are subjected to osmotic stress. The cytoplasm then becomes more viscous, a state necessary for vitrification (no crystallization) to occur (Sinniah & Gantait, 2013). Nevertheless, chemical toxicity and excessive osmotic stress may occur to the cells when overexposed to PVS2 (Hong, Yin, Shao, Wang, & Xin, 2009). Hence, it was essential to determine the most suitable dehydration period for G. hombroniana shoot tips to obtain sufficient dehydration for vitrification to occur and at the same time to avoid the toxic effects of the solution.
Stepwise dehydration seemed to be a better method for G. hombroniana shoot tips. In this method, a slower exposure to the full concentration of PVS2 is an alternative way to reduce the toxicity of the PVS2 exposure and the duration of PVS2 exposure can be extended. Shoot tips dehydrated with 50 % for 15 min followed by 100 % PVS2 for 10 min increased survival from 25 % to 41.67 %. Matsumoto and Sakai (2003) reported that the survival of Vitis shoots dehydrated for 30 min with 50 % PVS2 followed by 50 min with 100 % PVS2 increased from 60 % to 80 %. Higher survival and recovery were also reported by San José, Valladares, Janeiro and Corredoira (2014) when using two steps dehydration for cryopreservation of Alnus glutinosa (L.) Gaertn. shoot tips by vitrification. Hence, careful control of the dehydration procedure is important for a successful cryopreservation.
To enhance osmotolerance to PVS2, the shoot tips of G. hombroniana were precultured on solid medium with 0.3 M sucrose for one or two days. In several investigations, preculture treatment before cryopreservation was shown to increase the percentage survival of shoot tips. For example, the importance of preculture was clearly shown in the study by Chua and Normah (2011) where they reported that Nephelium ramboutan-ake shoot tips without preculture treatment gave poorer survival than those with two or three days of preculture. Similarly, low survival was observed from cryopreserved Garcinia cowa Roxb. shoot tips without preculture treatment (Yap, Noor, Clyde, & Chin, 2011). For the present study, cryopreserved shoot tips precultured for two days gave better survival of 75 % compared to only 50 % survival for one day preculture. Zhang et al. (2017) reported that by increasing the preculture duration in Helianthus tuberosus L. higher survival and regrowth percentage of shoot tips could be obtained. For a study on cryopreservation of lily, higher survival was also shown when the duration of preculture was two days (Chen et al., 2011). Preculture is important to assist the explants in getting ready for the vitrification process during dehydration (Niu, Zhang, Zhang, & Luo, 2010) and preculture duration has been shown to influence survival after cryopreservation (Ishikawa et al., 1997; Hong & Yin, 2009; San José et al., 2014).
For loading, addition of glycerol (1 M or 2 M) to 0.4 M sucrose improved survival with 45.83 % and 33.33 % respectively. Glycerol is considered a very efficient cryoprotectant as it imparts better membrane stabilization due to its small size and stereochemically oriented OH groups along one side of the molecule that resulted in better hydrogen bonding qualities with membrane phospholipids (Turner et al., 2001). Preculture treatment may not be sufficient to produce higher survival and direct exposure of less tolerant cells and shoot tips to PVS2 could be harmful to the cells due to osmotic stress and chemical toxicity (Pennycooke & Towill, 2000). Thus, loading is a step where the cells are preconditioned with cryoprotective solution before exposure to PVS2 (Langis & Steponkus, 1990; Sakai & Engelmann, 2007). An increase in glycerol concentration to 2 M slightly decreased the survival of G. hombroniana shoot tips. A study on G. cowa by Yap et al. (2011) showed similar results, where shoot tips treated with 1 M glycerol plus 0.4 M sucrose gave better survival and the addition of 2 M glycerol significantly reduced the survival from 81.7 % to 52.2 %. Yap et al. (2011) also suggested that the meristematic cells lose their structural integrity either due to high osmotic potential or due to the cytotoxicity effect when 2 M glycerol was added to the loading solution.
In this study, unloading using 0.4 M sucrose gave significantly higher survival (66.67 %) after cryopreservation compared to unloading with 1.2 M sucrose (25 % survival). After cryopreservation, unloading is necessary to dilute PVS2 solution, which becomes toxic to the cells if not removed (Kim et al., 2004). The main role of the unloading step is to avoid the detrimental osmotic shock, which would occur to the cryopreserved materials if they were transferred directly on to recovery medium (Kim et al., 2004). Hence, a gradual dilution of the dehydration solution is perhaps more preferred as shown where a dilute solution (0.4 M sucrose) gave better survival than a more concentrated one (1.2 M sucrose).
In the present study, water content decreased following each of the vitrification steps studied. Survival obtained was 41.67 % after PVS2 exposure at water content of 2.93 ± 0.28 g g-1 dw. In the case of G. mangostana (Ibrahim & Normah, 2013), the water content was 1.1 ± 0.3 g g-1 after exposure with PVS2 and the survival after cryopreservation was better. The shoot tips perhaps could be dehydrated to a much lower water content for better survival and would probably improve the survival for G. hombroniana after cryopreservation. Furthermore, from the observation throughout the experiments, shoot tips cryopreserved started to turn brown after six days on recovery medium, after which they slowly died. This may indicate that the water content has not reached the optimized level for shoot tip full recovery. Wesley-Smith, Vertucci, Berjak, Pammenter and Crane (1992) suggested that for plant samples to be cryopreserved successfully, water contents could be as high as 1.1-1.6 g g-1 dry mass. Hence further dehydration for G. hombroniana shoot tips is necessary.
The balance between all factors involved is essential and for G. hombroniana shoot tips this perhaps has not been achieved. Hence, further modification is needed in bringing down the water content without any detrimental effect. Vitrification steps especially the dehydration with PVS2 and perhaps recovery medium composition and condition need to be paid close attention to. It is also important to understand what caused the samples to decline in survival after cryopreservation by studying the stress related factors associated with cryo-injury. Nevertheless, this study has shown that G. hombroniana shoot tips could be cryopreserved by modification at various steps in the vitrification technique. The best steps involved preculture with 0.3 M sucrose for 48 h, loading with 2 M glycerol and 0.4 M sucrose for 20 min, followed by dehydration using stepwise PVS2 treatment (15 min with 50 % PVS2 followed by 10 min with 100 % PVS2 solution) at 0 °C and unloading with 0.4 M sucrose for 20 min. The finding is very significant towards germplasm conservation of this tropical recalcitrant species.












 uBio
uBio