Seeds of physic nut (Jatropha curcas L.) may present an oil content of approximately 50 %, representing a promising alternative for biodiesel production (Mendonça & Laviola, 2009). However, because the oil extracted from the seeds contains toxic components (e.g. curcin and phorbol esters), exploitation of the cake -a protein-rich, solid-phase subproduct of the oil extraction- as animal feed or organic fertilizer becomes compromised (Andrew, 2009).
The use of physic nut cake is of interest as feeding supplement for sheep, swine and other livestock, as well as for fertilization in agriculture, where it may generate important income and make these cultures more economically viable (Mendonça & Laviola, 2009).
In this sense, studies have been performed attempting to decrease the seed concentration of phorbol ester, considered the main toxic compound of physic nut (Begg & Gaskin, 2009), thus allowing the exploitation of the cake after oil extraction. In Brazil, Embrapa Agroenergy has been working on the breeding of physic nut varieties, aiming to reduce the phorbol ester content, and on processes that may help detoxify the subproduct. To test the efficiency of these two approaches, in vivo tests using animal models have been developed (Mendonça & Laviola, 2009). However, because phorbol ester is toxic, the tests usually lead to the feed depression of the animals tested and if the feeding is forced can cause death, bringing up an ethical problem concerning the assertion of success of the research.
Therefore, alternative models to investigate the toxic potential of physic nut varieties with different contents of phorbol ester should be considered. Among the tests available to study the toxic potential of a given compound or residue, plant models stand out for their good sensitivity and low cost (Leme & Marin-Morales, 2009), besides presenting good correlation with data from tests using animal models (Bianchi, Cabral-de-Mello, & Marin-Morales, 2015) or even human cells (Palmieri, et al., 2016).
Hence, this work aimed to evaluate the effect of physic nut oil from varieties considered toxic (containing known amount of phorbol ester) or non-toxic (without detectable phorbol ester), on meristematic cells of Lactuca sativa L. (lettuce) - a plant model employed to detect macroscopic alterations, via seed germination and root growth, as well as microscopic changes, involving cytogenetic bioassays to evaluate the mitotic index and occurrence of chromosomal and nuclear alterations (Silveira, Lima, Reis, Palmieri, & Andrade-Vieria, 2017). This way, efficiency of the plant model in distinguishing the studied varieties shall be verified, ultimately incorporating such tests in the toxicity analyses of different cultivars and in the validation of detoxication experiments, hence avoiding animal assays.
Material and methods
Plant material
Physic nut seeds - toxic variety CNPAE 1010 (4.21 mg/g of phorbol ester) and non-toxic variety CNPAE 1008 (undetected phorbol ester) - from the Active Germplasm Bank of Embrapa Agroenergy (Brazil) were kindly provided by the researcher Bruno Galvêas Laviola. The seeds were collected in November 2014 at the Rio Verde (GO, Brazil) and in July 2015 in Mexico. The experiment was conducted from May to July 2016.
Attainment of toxic and non-toxic oils and exposure of L. sativa seeds
Seeds of L. sativa L. (2n = 2x = 18) var. Lechuga Grandes (Isla Seeds) were acquired at agricultural supply stores and used as model for the assays. To obtain physic nut oil, one kilogram of seeds from each variety (toxic and non-toxic) were cold-pressed using a manual press for extraction of cold fixed oils (hydraulic, 15 tons, model similar to Ph15 Skay) (São José do Rio Preto, São Paulo, Brazil).
The experiment was conceived in a completely randomized design (CRD) comprising five repetitions, each consisting of one Petri dish (9 cm diameter) containing 50 seeds. The seeds were disposed onto filter paper moistened with 10 mL of each treatment solution. The concentrations of 22.5 %, 45 % and 67.5 % of emulsion (physic nut oil energetically mixed with distilled water) were evaluated, according to the IC50 determined by Andrade-Vieira, Botelho, Palmieri, Laviola, and Praça-Fontes, (2014). For each type of oil (toxic and nontoxic), emulsions were prepared at the three mentioned concentrations. Distilled water was used as negative control. In order to eliminate the effect of the oil’s lipoid condition on germination and plantlet development, an emulsion of water and soybean oil (45 %) from Cargill (São Paulo, São Paulo, Brazil) was also tested. The germination conditions followed Aragão et al. (2015).
Macroscopic analyses
The percentage of germinated seeds was assessed after 8, 16, 24, 36 and 48 hours of treatment. Root growth was determined after 48 hours of exposure, using a digital caliper from Digimess Instrumentos de Precisão Ltda (Mooca, São Paulo, Brazil). In accordance with the calculations described by Aragão et al. (2015), the following parameters were obtained: percentage of germination after 48 hours (GR), germination speed index (GSI), and root growth (RG).
Microscopic analyses
For microscopic analysis, at least 20 roots of each treatment were collected after measurement (item 2.3). The roots were fixed and the slides were prepared and evaluated as described Aragão et al. (2015). Five slides were evaluated per treatment, with approximately 500-800 meristematic cells analyzed per slide, and the different stages of mitotic division as well as possible chromosomal and nuclear alterations were recorded. The mitotic index (MI) were calculated as the number of dividing cells as a fraction of the total number of cells, and the frequency of chromosome (CA) and nuclear alterations (NA), expressed as the percentage of number of alterations divided by the total number of cells (Aragão et al., 2015).
The evaluated parameters (GR, GSI, RG, MI, CA and NA) were subjected to analysis of variance (α = 0.05), and the obtained mean values were compared by the test of KruskalWallis or Dunnett at 5 % significance level, as most suitable for each parameter. The statistical analyses were performed with the program R (R Development Core Team, 2011).
Results
Compared to the soybean oil, significant difference was observed in the percentage of germinated seeds (GR) and GSI in relation to water, demonstrating that the reductions in GR and RG in the treatments with physic nut oil are due to its chemical components (Table 1). Further, the RG in soybean oil was statistically similar to that in water.
Figure 1 gives an overview of the effects of the studied physic nut varieties (toxic and nontoxic) on the macroscopic parameters (GR, GSI and RG). For both oils, significant reduction in GR was obtained at the highest concentrations (45.0 % and 67.5 %). Regarding GSI and RG, all tested concentrations differed from water, whether toxic or non-toxic variety (Table 1). For the first tested concentration of 22.5 % of each oil (JC11 being non-toxic and JC21 being toxic), both varieties presented similar effects on the three evaluated parameters (Figure 1, Table 1). In the second tested concentration (45 %), the toxic oil caused a reduction 32 % greater in GR than the non-toxic oil. For this same parameter, at the highest concentration (67.5 %) the reduction increased to 90 % for the toxic in relation to the non-toxic oil (Table 1). This pattern of 30-40 % reduction for toxic in relation to non-toxic oil at the second concentration, and of 80-90 % at the highest concentration, was observed in the three assessed macroscopic parameters (Figure 1, Table 1).
Table 1: Effects of physic nut oil over the germination and plants growth on the plant model Lactuca sativa
| - | GR (%) | - | - | GSI | - | RG (mm) | ||
|---|---|---|---|---|---|---|---|---|
| Treatments | Mean | SD | Mean | - | SD | Mean | SD | |
| Negative control | 99.2 | 3.30 | 24.57 | - | 0.80 | 8.60 | 1.86 | |
| JC11 | 99.6 | 2.10 | 16.55* | - | 1.10 | 6.73* | 1.10 | |
| JC12 | 52.8* | 1.30 | 6.75* | - | 1.70 | 2.57* | 1.30 | |
| JC13 | 10.8* | 0.82 | 1.13* | - | 0.38 | 0.31* | 0.03 | |
| JC21 | 98.8 | 3.10 | 15.55* | - | 1.80 | 5.39* | 1.52 | |
| JC22 | 36* | 1.45 | 4.24* | - | 1.30 | 1.53* | 0.31 | |
| JC23 | 1.2* | 0.32 | 0.13* | - | 0.02 | 0.06* | 0.02 | |
| Soybean oil | 99.6 | 2.89 | 23.50 | - | 1.24 | 8.41 | 2.12 | |
Abreviations means: GR - Germination Ratios; GSI - Germination Speed Index; RG - Root Growth; SD - Standard Deviation. The treatments JC11, JC12 and JC13 refeer to the non-toxic oil at the concentrations: JC11 = 22.5 %; JC12 = 45 % and JC13 = 67.5 %. The treatments JC21, JC22 and JC23 refeer to the toxic oil and the following concentrations: JC21 = 22.5 %; JC22 = 45 % and JC23 = 67.5 %. Means followed by * are statistically different from the negative control (distilled water) by Dunnet’s Test p< 0.05.
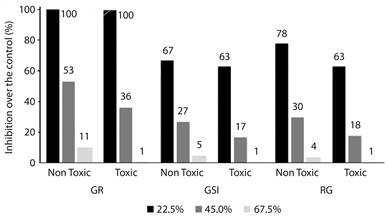
Figure 1 Inhibitory effects of physic nut toxic and non-toxic oil at the concentrations of 22.5 %, 45 % and 67.5 % of emulsion over the the germination and seedlings growth on the plant model Lactuca sativa. GR - Germination Ratios; GSI - Germination Speed Index; RG - Root Growth.
Here, observing the MI of meristematic cells of L. sativa (Table 2), a significant reduction in dividing cells was verified for the treatments with toxic and non-toxic physic nut oil in relation to water or soybean oil. The non-toxic oil inhibited the MI of the exposed cells from 17 % to 26 % in comparison to water, whereas the toxic oil caused a reduction from 52 % to 70 % (Table 2).
Table 2: Effects of physic nut oil over the cell cycle of root tips cells from the plant model Lactuca sativa
| - | MI (%) | - | CA (%) | - | NA (mm) | ||
|---|---|---|---|---|---|---|---|
| Trataments | Mean | SD | Mean | SD | Mean | SD | |
| Negative Control | 7.11a | 1.23 | 0.00a | 0.00 | 2.55a | 0.43 | |
| JC11 | 5.94c | 1.96 | 0.05a | 0.01 | 1.37a | 0.82 | |
| JC12 | 5.90c | 1.74 | 0.09a | 0.04 | 1.53a | 0.95 | |
| JC13 | 5.29c | 1.47 | 0.07a | 0.06 | 4.91a | 1.59 | |
| JC21 | 3.34d | 1.67 | 0.12a | 0.07 | 2.23a | 0.79 | |
| JC22 | 3.39d | 1.62 | 0.08a | 0.05 | 5.91a | 1.13 | |
| JC23 | 2.19e | 0.74 | 0.13a | 0.05 | 2.26a | 1.73 | |
| Soybean Oil | 9.30b | 1.94 | 0.04a | 0.02 | 4.28a | 1.58 | |
Abreviations means: MI - Mitotic Index; CA - Chromosome Alteration; NA - Nuclear Alteration; SD - Standard Deviation. The treatments JC11, JC12 and JC13 refeer to the non-toxic oil at the concentrations: JC11 = 22.5 %; JC12 = 45 % and JC13 = 67.5 %. The treatments JC21, JC22 and JC23 refeer to the toxic oil and the following concentrations: JC21 = 22.5 %; JC22 = 45 % and JC23 = 67.5 %. Means followed by different letter at the column are statistically different from each other by Kruskal Wallis Test p< 0.05.
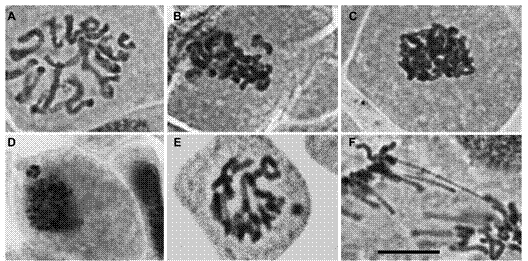
Figure 2 Exemple of the main cell cycle alterations observed on the root tip cells of Lactuca sativa exposed to physic nut oil (toxic and non-toxic). (A) C-metaphasis, note that chromosomes are not aligned at the equatorial cell plate; (B) non-oriented chromosomes in relation the metaphasic plate; (C) sticky chromosomes in metaphasis; (D) micronuclei in interphase; (E) micronuclei in prophasis; (F) anaphasis bridge. The images were captured on the 40X objective under the Olympus BX60 microscope. Bar = 10 µm.
The possible chromosomal alterations due to toxicity of phorbol ester were also assessed (Table 2). All treatments induced cell cycle alterations (CCA). The frequency of CCA in water was 0 %, whereas in the treatments it varied from 0.05 % (lowest concentration of the non-toxic oil) to 0.13 % (highest concentration of toxic oil), without statistical differences to the control (water) (Table 2). Among the verified alterations, the most frequent were chromosomes mis-orientation in the mitotic spindle, adhered chromosomes, bridges, and micronuclei (Figure 2).
Discussion
Lettuce is an excellent model for macroscopic analyses, as it allows the evaluation of adverse events of a toxic compound on germination and root growth from the early initial phases of seed development in according to Valerio, García and Peinado (2007). The statistical differences regarding the dynamics of germination and growth of roots exposed to physic nut oil was obtained after 48 hours of exposure, showing that, when present, the toxicity response is rapid. Moreover, the applied test requires little time, is simple and of low cost, which corroborates the perception of other authors on the use of macroscopic tests to investigate the toxic effects of any simple substance or chemical compound (Palmieri, Luber, Andrade, & Davide, 2014; Silveira, et al., 2017). Pinheiro et al., (2015) investigated the essential oil action of leaves of Plectranthus amboinicus, Carvacrol, and Thymol on lettuce, observing the toxic action of these compounds on seed development. Lettuce was also used to investigate the cytogenotoxic effect of the ethanolic extract of leaves of Annona crassiflora, with a significant reduction in seed germination (Ribeiro et al., 2013).
In addition, it was observed that the presence of compounds like phorbol ester in the physic nut oil, even in varieties considered non-toxic by chemical analysis due to presenting low content of the ester, is sufficient to significantly inhibit both germination and root growth of developing plantlets. Andrade-Vieira et al. (2014) attributed the inhibitory effect of physic nut on root growth to phorbol ester, via mechanisms related to activation of proteins that hamper the progression of the cell cycle, demonstrated by inhibition of MI. These data strengthen the discussion of Andrade-Vieira et al. (2014) that the presence of phorbol ester, a diterpene analogous to diacylglycerol (King, et al., 2009), hampers the transition of the cell cycle, maintaining the activation of the protein kinase C for a longer period.
In accordance to Andrade-Vieira, et al., (2014) the toxicity of J. curcas seeds has been examined with various bioassays by using animals such as cattle, goats and fish as test systems. The goals of such bioassays lie in the interests of using the plant seed for biodiesel production and in animal nutrition, and hence, in making its use more economically viable. However, if the seed is toxic it can lead to the death of the animals. Therefore, tests with lettuce may be an alternative for such purposes.
With the results obtained in the present study, it is possible to note that the tests carried out with L. sativa reveal the toxicity potential of the J. curcas seeds, and confirmed that the phytotoxic and mitodepressive effect of phorbol ester is dependent on its concentration and availability to the cells. In the toxic varieties, where it is present in amounts considered high (4.21 mg of phorbol ester per 1 g of seed) (Makkar, Becker, Sporer, & Wink, 1997), this mitodepressive effect is more pronounced with increasing concentration of the oil and, consequently, of phorbol ester. This becomes evident when comparing the MI of toxic and non-toxic oil (i.e. without phorbol ester): the toxic oil inhibits cell division by 50 % more than the non-toxic type. In addition, according to Fiskejö (1993), only mitodepressive effects above 50 % indicate toxicity of a compound. This way, only the oil considered toxic was effectively cytotoxic.
The alterations classified as aneugenic, related to malfunctioning of the mitotic spindle, including non-oriented chromosomes and c-metaphases (Leme & Marin-Morales, 2009), were the most frequent in previous studies carried out by Andrade-Vieira et al. (2014), who showed for the first time, the application of a plant model test to investigate the effect of physic nut oil. Corroborating the data obtained by those authors, here the presence of adherent chromosomes was also recurrent, reinforcing the cytotoxicity of physic nut oil and its effects on cell proteins (Andrade-Viera, et al., 2014). The presence of condensed nuclei, which may be related to cell death process (Andrade-Vieira, Gedraite, Campos, & Davide, 2011), was also detected in this work, though the treatments did not differ statistically from the control.
In conclusion, the toxic potential of the physic nut oil was demonstrated by the reduction in macroscopic parameters like GR and RG and microscopic criteria such as MI; thus, the oil can be classified as phytotoxic and cytotoxic. Moreover, the toxic oil, with known higher content of phorbol ester, presented more pronounced phyto- and cytotoxic effects than the non-toxic oil, which has undetected phorbol ester content. In this way, the phytotoxic and cytogenetic bioassays distinguished toxic and non-toxic varieties from J. curcas. This tool can help identify non-toxic varieties in order to verify the efficiency of detoxification. The assessed parameters, especially RG and MI, were efficient in the investigation of physic nut oil toxicity, allowing the distinction between toxic and non-toxic varieties at any analyzed concentration. Hence, the evaluation of root growth and cell division in the plant model L. sativa can be proposed as an alternative to animal tests to distinguish the varieties with high and low phorbol ester concentration.












 uBio
uBio 

