Plasticity in organs is crucial to optimize physiological efficiency and therefore maximize the reproductive output of an organism under changes in food availability and density of individuals (Levitan, 1991). Sea urchins allocate the energy in different organs depending generally on the availability of food (Hill & Lawrence, 2003). Most sea urchins allocate more resources to the Aristotle’s lantern (the feeding apparatus) when food is scarce; the lantern becomes relatively larger at a low food availability to increase the strength of scraping. Therefore the difference in the size of the lantern relative to the test diameter is an adaptive morphological plasticity (Ebert, 1980; Ebert et al., 2014). Laboratory studies showed that when food is limiting, more growth occurs in the demi-pyramids (referred to jaw) compared to the test (Levitan, 1991). On the other hand, when food availability is high, sea urchins present relative small lanterns and large gonads (Black, Codd, Hebbert, Vink & Burt, 1984; Fernández & Boudouresque, 1997; Ebert et al., 2014). However, the relative size of de Aristotle´s lantern not always differs between populations (Ebert & Russel, 1992; Lawrence et al., 1996), which could be related to the species life-history strategy since in sea urchins species with stress tolerant strategy the plasticity tends to be minimal (Lawrence, 1990).
To evaluate the changes in the Aristotle’s lantern, the frequently chosen method has been the classic morphometric by using the length of the jaw (Ebert, 1980; Levitan, 1991, 1992; Brey, Pearse, Basch, Clintock & Slattery 1995; Lawrence et al., 1996; McShane & Anderson, 1997; Wing, Gibbs & Lamare, 2003; Hagen, 2008; Pederson & Johnson, 2008; Hernández & Russell, 2010; Ebert et al., 2014), the length of the entire lantern (Black et al., 1982, 1984; Arnedo & Ibañez, 1985) or the lantern index (Edwards & Ebert, 1991; Fernández & Boudouresque, 1997; Garrido, 2003; Hill & Lawrence, 2003) as a variable. Classic morphometric focuses on a linear measurement, which impairs analyzing the presence of changes in the shape of the structure (Bookstein et al., 1985; Adams, Rohlf & Slice, 2004; Zelditch et al., 2004). Geometric morphometrics analysis solves these difficulties by analyzing specific points (landmarks) of a structure, making possible to focus on the shape changes (Rohlf, 1998; Richtsmeier et al., 2002; Adams et al., 2004). Given the importance of the structures of the Aristotle’s lantern (jaw and rotula) in the feeding process of the sea urchins, it is important to analyze the morphological variation in length and shape of these structures.
Arbacia dufresnii (Blainville, 1825) is the most common sea urchin in the South Atlantic Ocean that inhabits both coasts of South America and it is the only species of the genus that it is not distributed exclusively in neotropical regions (Lessios et al., 2012; Wangensteen, 2013). Arabcia dufresnii has been described as a carnivore (Penchaszadeh & Lawrence, 1999), as an omnívorous in San Jorge gulf (Díaz de Vivar et al., 2012), and as an herbivore in Nuevo gulf and San José gulf (Galván et al., 2009). The different feeding habits would indicate different food availability along the patagonian coast and behavioral and morphological plasticity in the feeding habit of the species.
The aim of this study was to analyze the morphological plasticity of two structures of Aristotle´s lantern (jaw and rotula) of A. dufresnii populations inhabiting different patagonian gulfs (San Matías, San José, Nuevo and San Jorge) by using both classic and geometric morphometric to further discuss its relation with different food availability at each environment.
Materials and methods
Study areas
The study was conducted in the patagonian gulfs of Argentina: San Matías, San José, Nuevo and San Jorge, ranging from 40°50ˈ to 47° S. Selected sites present different environmental characteristics, either mussel beds or disturbed areas by the invasive alga Undaria pinnatifida. The San Matías Gulf (SMG) is a semi-enclosed basin, partially conected with the open sea through a shallow sill (60 m depth) (Rivas & Beier, 1990). Its surface is 19 700 km2 and has a maximum depth of 180 m (Mazio & Vara, 1983). The samplings were made in El Sótano (ES; 40º 56’ 30’’ S - 65º 6’ W), on NW coast. Bottom sediment is dominated by sand near the coast line and gradually mixed with shell hash, gravel, and mud (Escofet, Orensanz, Olivier & Scarabino, 1977; Morsan, 2008). This area is characterized by a soft bottom community dominated by bivalves and with scarce algae (Doldan, 2013).
San José Gulf (SJG) is located on the northern margin of Valdés Peninsula. It opens to SMG through a 6.9 km wide mouth located on its northwestern margin. Its surface is 817 km2 with a mean depth of 40 m and maximum depth of 80 m (Amoroso & Gagliardini, 2010). Two sampling sites were selected in this gulf: Punta Tehuelche (PT; 42º 23’ S - 64º 17’ W) and Zona 39 (Z39; 42º 23’ S - 64º 04’ W). Both areas have similar hard bottom communities with abundance of macroalgae, there are shallow rocky reefs of limestone platforms (Zaixso et al., 1998; Boraso de Zaixso, Zaixso & Casas 1999). SJG has been invaded by the alien alga U. pinnatifida in 2004 (Irigoyen, 2009). Currently, U. pinnatifida dominates the PT area along with small mussels. Instead in Z39 the algae Codium sp. is the dominant one (Martelli et al., unpublished).
The Nuevo Gulf (NG), located on the southern margin of Valdés Peninsula, is an elliptical basin with a surface of 2 440 km2 and a maximum depth of 184 m that connects to the continental shelf through a 17 km wide gap (Mouzo et al., 1978). Punta Cuevas (PC, 42º 46’ 44’’ S - 64º 59’ 52’’ W), located near Puerto Madryn city, is a shallow rocky reef of limestone platforms (Irigoyen,Trobbiani, Sgarlatta & Raffo 2011) that has been invaded by U. pinnatifida for over 20 year (Casas & Piriz, 1996). Benthic community was dominated by the algae Codium spp., Dyctiota sp. and Ulva spp. before the invasion of U. pinnatifida (Piriz et al., 2003). After the invasion, every late winter and spring a dense forest of the invasive alga dominates the rocky reef (Casas et al., 2008). Its presence is associated with a dramatic decrease in species richness and diversity of native seaweeds (Casas, Piriz & Scrosati, 2004).
The San Jorge Gulf (SJOG), it is the southern patagonian gulf, which is the largest one with a surface area of 39 340 km 2. Its maximum depth is almost 110 m (Akselman, 1996) and open to waves entering from the Atlantic Ocean (Isla, Iantanos & Estrada, 2002). La Tranquera beach (LT, 46º 02’ 33’’ S - 67º 35’ 52’’ W) is a high energy rocky coast located in the central area of the gulf. There are extensive kelp forests of Macrocystis pyrifera in almost all the rocky sublittoral in the sampling area, with low incidence of the recently introduced U. pinnatifida (Zaixso et al., in press).
Sample Processing
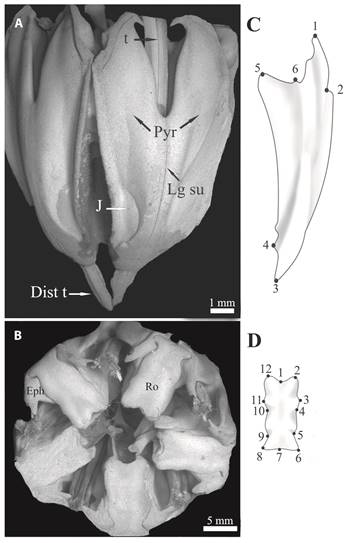
Sea urchins were randomly collected by scuba diving at each site (ES = 29, PT = 30, Z39 = 41, and PC = 31). In the laboratory, all individuals were narcotized by immersion for 15 min in 5 % MgCl2 in filtered seawater before dissection. Each sea urchin was blotted dry and wet mass and test diameter were determined. The Aristotle’s lantern of A. dufresnii (Fig. 1) was dissect out, weighed and measured without disarticulating as proposed by Black et al. (1982). Lantern’s soft tissue was removed by immersion in 5 % sodium hypochlorite for 24 h. Then, the lantern elements were thoroughly rinsed in tapwater and air dried. One jaw (ES = 29, PT = 30, Z39 = 41, PC = 31, LT = 31) and one rotula (ES = 18, PT = 30, Z39 = 31, PC = 29, LT = 31) were selected from each lantern to analyze the morphological variation. Photographs were taken on each structure with a digital camera SONY DSC-W70 (7.2 Megapixels) mounted on a table top to ensure parallelism between the focal plane of the camera and frontal plane of the structure. Each jaw was placed with the inner side facing up to. Each photograph included a scale to standardize the structure sizes. In order to conduct classic morphometric analysis the linear length of jaw and rotula were obtained from the photographs using IMP (Integrated Morphometrics Package) software. Lengths were estimated from landmarks 3 to 6 and from 1 to 7 for jaw and rotula, respectively (see below, Figs. 1C, 1D). In order to conduct geometric morphometric analysis, lanterns from LT were incorporated to the analysis. Individual test diameters of sea urchin collected at LT were not available, however, the lantern were included in the geometric morphometric analysis because the urchins of this population presented almost twice test diameter than the other populations (ca. 50 mm, Epherra et al., 2014). For this analysis, six type-I landmarks arranged in two dimensions were used to characterize jaw shape (Fig. 1C). The rotula was placed with the oral surface facing up and 12 type-I landmarks arranged in two dimensions were selected to characterize structure shape (Fig. 1D). In all cases, landmark coordinates were obtained by using the software TPSDig version 2.
Data analysis
To test whether sea urchin Aristotle’s lantern morphology measurements (lantern height, lantern weight, length of jaw and length of rotula) (dependent variables) varied between populations, test diameter or the presence of the invasive kelp U. pinnatifida (extrinsic variables) a generalized least square (GLS) model was performed. Since the Aristotle’s lantern measurements data presented variance heterogeneity per site and in the presence or absence of U. pinnatifida, the variance structure with different spread per stratum (“VarIdent” Variance Structure) was included into GLS analyses. Variance structure considers that each factor’s stratum has a different spread, modeling it on each case (for more detail see Zuur et al., 2009). Models with different number and combinations of the explanatory variables were fitted by Maximum Likelihood and the performance of each model was assessed by Information Theory (IT) procedures. To obtain the best model, Akaike´s information criterion (AIC) was applied. AIC differences (∆ i ) and normalized weights of AIC (w i ) of all possible models were computed (Burnham, Anderson & Huyvaert, 2011; Symonds & Moussalli, 2011). In addition, the 95% confidence intervals for parameters estimated in the best model we calculated. Statistical analyses were performed by using the Open Access Software R 3.0.2 (R Development Core Team 2013). The function “gls” from the “nlme” package (Pinheiro et al., 2013) and the “bbmle” library (Bolker and R Development Core Team 2013) were used.
Shape changes in jaw and rotula were visualized through relative warp (RW) analysis. A principal component analysis was performed over the uniform and non-uniform components of variation, where RW are the principal component orthogonal axis used to describe the main tendencies in shape variation between specimens within a sample (Bookstein, 1998). TpsRelw software Version 1.44 was used to translate, rotate, and scale the landmark configurations for each structure by using the generalized least squares (GLS) superimposition method. Translation and rotation was achieved by superimposing the landmark configurations and adjusting their individual inclination and relative position by minimizing the square root of the sum of squared differences between corresponding landmarks (Rohlf, 1999). Scaling was performed by correcting the landmark configurations in such way that all present the same centroid size. The program was also used to calculate the average individual map (consensus configuration), to derive the uniform (that affecting to the same extent all of the landmarks of the form under study) and nonuniform (all other landmark local differences) components of variation, and to estimate size of structures as the centroid size (the square root of the sum of the squared deviations of landmarks from a centroid point) (Rohlf, 1998; Richtsmeier et al., 2002).
Results
Changes in size
The relations between length of the jaw and the rotula and test diameter at the population are shown in Table 1. The length variation of the jaw and the rotula was best described by the minimal adequate generalized least square model (GLS) that incorporated the population and test diameter as explanatory variables. There were differences between populations for all Aristotle’s lantern morphological measurements (lantern height, lantern weight, length of jaw and length of rotula). Moreover, the AIC indicates that the variance structure “VarIdent” improved the model compared to the linear regression model.
Table 1 Generalized Least Square Models selection explaining sea urchin Aristotle’s lantern morphology measurements variation due to test diameter and between sampled populations
| Model | N° par i | lantern height | lantern weight | length of jaw | length of rotulae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIC | ∆ i | w i | AIC | ∆ i | w i | AIC | ∆ i | w i | AIC | ∆ i | w i | ||
| Pop+ Diam | 6 | - 86.88 | 10.45 | 0.01 | 111.84 | 6.27 | 0.04 | 39.26 | 2.4 | 0.22 | -203.07 | 103.19 | < 0.001 |
| Pop + Diam + var | 9 | - 97.34* | 0 | 0.99 | 105.57* | 0 | 0.94 | 36.87* | 0 | 0.72 | 306.26* | 0 | 0.99 |
| Diam + var | 6 | - 82.38 | 14.96 | < 0.001 | 113.65 | 8.08 | 0.02 | 74.45 | 37.59 | < 0.001 | - 282.92 | 23.34 | < 0.001 |
| Pop + var | 8 | 66.68 | 164.02 | < 0.001 | 217.53 | 111.96 | < 0.001 | 41.8 | 4.94 | 0.06 | - 186.46 | 119.8 | < 0.001 |
| null + var | 5 | 60.94 | 158.27 | < 0.001 | 223.33 | 117.76 | < 0.001 | 72.26 | 35.59 | < 0.001 | - 160.58 | 145.68 | < 0.001 |
| null | 2 | 67.62 | 164.96 | < 0.001 | 237.4 | 131.83 | < 0.001 | 75.41 | 75.41 | < 0.001 | - 149.39 | 156.87 | < 0.001 |
N° par i , number of parameters; AIC, Akaike´s information criterion; ∆i, AIC differences; w i , normalized weights of AIC.
Pop, population; Diam, test diameter; var, “VarIdent” variance structure.
* indicates the best models.
The jaw length was similar between PC and PT, ES and PT and ES and Z39. The lantern height, lantern weight and the rotula length were similar between ES and Z39 and ES and PT (Table 2). Nonetheless, differences between some populations were found, PC presented larger jaw values than ES and Z39, and PT jaw lengths were larger than Z39 (Table 2; Fig. 2).
Table 2 Parameter estimations for the selected Generalized Least Square Models explaining sea urchin Aristotle’s lantern morphological measurements variation due to test diameter and between sampled populations
| - | - | Estimate | - | CI |
|---|---|---|---|---|
| Response Variable | Coefficients | ± SE | Lower | Upper |
| lantern height | Intercept | 0.23 ± 0.06 | 0.11 | 0.34 |
| - | Pop - PT | 0.01 ± 0.06 | - 0.1 | 0.12 |
| - | Pop - ES | -0.07 ± 0.05 | - 0.17 | 0.03 |
| - | Pop - Z39 | -0.16 ± 0.05 | - 0.26 | - 0.06 |
| - | Diam | 0.30 ± 0.02 | 0.27 | 0.33 |
| lantern weight | Intercept | - 0.82 ± 0.11 | - 1.04 | - 0.6 |
| - | Pop -PT | 0.43 ± 0.11 | 0.22 | 0.64 |
| - | Pop -ES | 0.17 ± 0.09 | - 0.01 | 0.34 |
| - | Pop -Z39 | 0.09 ± 0.08 | - 0.06 | 0.25 |
| - | Diam | 0.57 ± 0.04 | 0.49 | 0.64 |
| length of jaw | Intercept | 0.90 ± 0.09 | 0.72 | 1.08 |
| - | Pop - PT | - 0.10 ± 0.07 | - 0.25 | 0.04 |
| - | Pop - ES | - 0.30 ± 0.07 | - 0.45 | - 0.16 |
| - | Pop - Z39 | - 0.47 ± 0.07 | - 0.61 | - 0.32 |
| - | Diam | 0.06 ± 0.03 | 0.01 | 0.12 |
| length of rotulae | Intercept | 0.02 ± 0.01 | - 0.004 | 0.04 |
| - | Pop - PT | 0.04 ± 0.01 | 0.02 | 0.06 |
| - | Pop - ES | 0.04 ± 0.02 | 0.01 | 0.08 |
| - | Pop - Z39 | 0.08 ± 0.03 | 0.03 | 0.13 |
| - | Diam | 0.10 ± 0.004 | 0.1 | 0.11 |
CI, 95 % confidence intervals;
ES, El Sotano; Z39, Zona 39 PT, Punta Tehuelche; PC, Punta Cuevas.
The parameter for Pop (Population) is given as relative to Punta Cuevas population.
The minimal adequate GLS model incorporated the presence of Undaria pinnatifida as a explanatory variable to account for the differences in jaw length, lantern height and rotula length between the populations (Table 3). The presence of U. pinnatifida was related to larger values of length of jaw (Fig. 2) and lantern height. However, the rotulae were larger in the absence of the invasive alga and the lantern weight did not differ in the presence or absence of U. pinnatifida (Table 3).
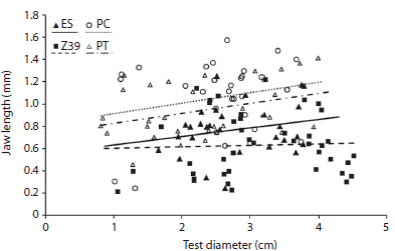
Fig. 2 Relationship between jaw length (mm) and test diameter (cm) between the selected sites: El Sotano (ES), Zona 39 (Z39), Punta Tehuelche (PT) and Punta Cuevas (PC). White symbols correspond to the presence of Undaria pinnatifida; black symbols represent the sampled sites without U. pinnatifida. The lines indicate the estimated mean of generalized least square model.
Table 3 Generalized Least Square Models selection explaining sea urchin Aristotle’s lantern morphology measurements variation due to the presence of invasive algae Undaria pinnatifida and test diameter
| Model | N° par i | lantern height | lantern weight | jaw length | length of rotulae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIC | ∆ i | w i | AIC | ∆ i | w i | AIC | ∆ i | w i | AIC | ∆ i | w i | ||
| Und + Diam | 4 | - 85.85 | 9.99 | 0.01 | 126.6 | 3.39 | 0.12 | 42.65 | 1.94 | 0.24 | -203.62 | 60.97 | < 0.001 |
| Und + Diam + var | 5 | - 95.84 | 0 | 0.99 | 125.42* | 2.21 | 0.22 | 43.65 | 2.94 | 0.14 | -264.6* | 0 | 0.79 |
| Diam + var | 4 | - 85.36 | 1.48 | < 0.001 | 123.21 | 0 | 0.66 | 77.91* | 37.2 | < 0.001 | -261.91 | 2.68 | 0.21 |
| Und + var | 4 | 60.86* | 156.7 | < 0.001 | 236.26 | 113.05 | < 0.001 | 40.71 | 0 | 0.62 | -174.28 | 90.32 | < 0.001 |
| null + var | 3 | 59.34 | 155.18 | < 0.001 | 237.88 | 114.67 | < 0.001 | 75.41 | 34.69 | < 0.001 | -148.47 | 116.13 | < 0.001 |
| null | 2 | 67.62 | 163.47 | < 0.001 | 237.4 | 114.19 | < 0.001 | 75.95 | 35.23 | < 0.001 | -149.39 | 115.2 | < 0.001 |
N° par i , number of parameters; AIC, Akaike´s information criterion; ∆i, AIC differences; w i , normalized weights of AIC.
Pop, Undaria pinnatifida; Diam, test diameter; var, “VarIdent” variance structure.*indicates the best models.
Change in shape
Relative warp analysis showed that the first and second relative warps explained more than 80 % of the total shape variation in both cases (RW1 61 % and RW2 19 % for jaw; RW1 68 % and RW2 17 % for rotula). There was a large overlap in shape of sea urchins from different populations. As a general pattern, jaw shape changed gradually along RW1 mainly in the aboral region. Negative magnitudes of RW1 were associated to an elongated aboral tip of jaw (Fig. 3A). The shape of the jaw did not change with increasing centroid size (Fig. 3B). The main change in the rotula through the RW1 was along the longitudinal axis of the structure, the positive values were associated to a more elongated rotula (Fig. 4B). The rotula shape changed as centroid size increased (Fig. 4B). Sea urchins from all the populations presented a similar pattern of shape change of the rotula; larger sea urchins presented a more elongated overall shape than smaller sea urchins.
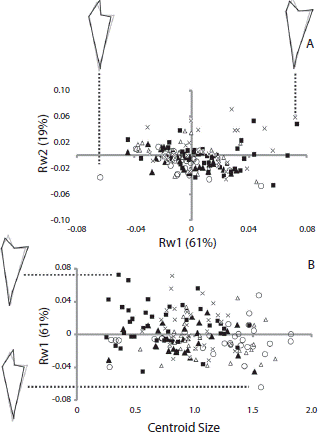
Fig. 3 (A) Scatterplots of the first and second components of the relative warp analysis (RW1 and RW2 respectively) using jaw of Arbacia dufresnii and (B) of the centroid size and first component of the relative warp analysis. Filled squares correspond to individuals from the location Zona 39, filled triangles correspond to individuals from the location El Sótano, empty triangles correspond to individuals from the location Punta Tehuelche, empty circles correspond to individuals from the location Punta Cuevas, and crosses correspond to individuals from the location La Tranquera. In parenthesis is expressed the percentage of the total shape variation explained by each component or the relative warp analysis. On RW1 axis is represented the shape of jaw at the extremes values of the axis.
Discussion
In Arbacia dufresnii the relative length of the jaw was the classical measurement that best reflected the changes between populations. The lantern weight and lantern height did not separate populations, because the minimal adequate GLS revealed a large values overlap. The geometric morphometric analysis of the structures of the Aristotle’s lantern of A. dufresnii showed that the shape of the jaw did not vary among populations or with the increase in diameter of the sea urchins. Given that the jaws grow on all surfaces (Ebert, 1982), it appears that both ends of the lantern of A. dufresnii are growing at the same time therefore the shape of the jaw does not change. The rotula shape did not change between populations; however, it did change with the increase of the diameter of the sea urchins. Considering that the rotulae articulate the jaws in the aboral face, bigger jaws should have more elongated rotulae, therefore the changes in shape would be explained by the growth of the lantern.
Energy allocation plasticity in the lantern appears to be related to food availability. A larger lantern would increase the strength of scraping (Ebert, 1980), therefore increasing the grazing potential, which is likely to be of adaptive significance in resource-limited environments (Black et al., 1984). The genus Arbacia has been described as omnivorous with a strong tendency to carnivory (Vásquez et al., 1984; Fernández & Boudouresque, 1997; Penchaszadeh & Lawrence, 1999; Hill & Lawrence, 2003; Cobb & Lawrence, 2005; Wangsteen et al., 2011); the sea urchins have a wide range of food item and due to the ability to feed on encrusting algae and protected animals with hard calcareous shells, it has been suggested that their large Aristotle’s lantern is indicative of a durophagic habit (Gianguzza & Bonaviri, 2013). Similarly, Hagen (2008) studied two different sympatric species of the genus Strongylocentrotus and postulated that the enlargement in size of the lantern is a functional specialization for durophagy (ability to exploit hard shelled prey). More specifically, in the genus Arbacia, A. lixula may change their feeding habit in relation to the availability of food in the field; when algae are low they can be carnivorous and when algae are abundant are usually omnivorous (Cobb & Lawrence, 2005). In ES, an area with scarce algal coverage (Doldán, 2013), A. dufresnii would feed on the spat of bivalves that are abundant (Penchazadeh & Lawrence, 1999).
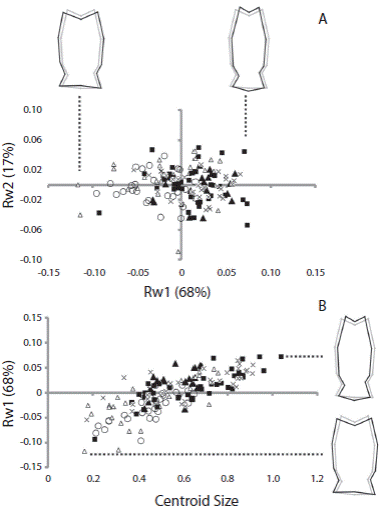
Fig. 4 (A) Scatterplots of the first and second components of the relative warp analysis (RW1 and RW2, respectively) using rotula of Arbacia dufresnii and (B) of the centroid size and first component of the relative warp analysis. Filled squares correspond to individuals from the location Zona 39, filled triangles correspond to individuals from the location El Sótano, empty triangles correspond to individuals from the location Punta Tehuelche, empty circles correspond to individuals from the location Punta Cuevas, and crosses correspond to individuals from the location La Tranquera. In parenthesis is expressed the percentage of the total shape variation explained by each component or the relative warp analysis. On RW1 axis is represented the shape of rotulae at the extremes values of the axis.
In fact, a study of its diet in this area indicated that they are predators (Rubilar et al. in prep). On the other hand, sea urchins from ES did not present larger jaws as expected. Therefore, the hypothesis that enlarged lantern size is an adaptation for durophagy may be useful to compare between species rather than among populations. Examples of this can be found in the data published comparison among species (e.g. Contreras & Castilla, 1987; Hagen, 2008; Bonaviri et al., 2011; Agnetta et al., 2013).
In both SJG and NG populations of A. dufresnii present an omnivorous habit with tendency to hervibory (Galvan et al, 2009; Castro, 2014). The analysis of the relative jaws length showed that, although in these three populations the sea urchins tend to have an herbivorous habit, the range of length jaw was large. The relative jaw length has been a useful way to infer the food availability in field populations of sea urchins (Ebert 1980, Levitan 1991, Fernández & Boudouresque, 1997; McShane & Anderson, 1997; Pederson & Jonhson, 2008; Ebert et al., 2014). Therefore, food availability may be responsible for the differences in jaw length among populations of SJG and NG.
Both areas inside GSJ, PT and Z39, are characterized by high abundance of macroalgae (Boraso de Zaixso et al., 1999), however, both populations presented different relative jaw length. Sea urchins with the smallest jaw were found in Z39 population, which would indicate the highest food availability of the populations under study. According to Castro (2014) sea urchins from Z39 present a more diverse and stable diet all year round. Therefore, differences in diet may be responsible for the differences in jaw length found between these two populations.
Sea urchins from areas invaded by Undaria pinnatifida, PC and PT, presented similar relative jaw length; these lanterns were the larger ones. This would indicate that these areas presented the lowest food availability among the studied populations of A. dufresnii. Even though, U. pinnatifida would be a new food item, does not imply that availability of food increases. According to Casas et al. (2004), invasive macroalgae presence is associated with a decrease in species richness and diversity of native macroalgae. In addition, A. dufresnii feeds on U. pinnatifida only during the summer when the macroalgae is rotten (Teso, Bigatti, Casas, Piriz & Penchaszadeh, 2009; Castro, 2014). Moreover, the presence of U. pinnatifida produces an increment in the density of A. dufresnii in natural environment (Irigoyen et al., 2011), Therefore, U. pinnatifida favors a scenario in which the availability of food is decreased due to the competition between sea urchins and declining of richness of native algae, creating an environment similar to the barrens that are characterized by low food availability (Pederson & Johnson, 2008).
Despite the lack of direct evidence, the differences found in the relative jaw length among the populations under study appear mainly related to differences in food availability, and indirectly influenced by the presence of U. pinnatifida. However, other factors such as density (Black et al. 1982; Garrido, 2003), feeding habit or preference, and reproductive cycle may be also important to evaluate. The results found in this study along with the differences found in reproductive traits (Epherra et al., 2014) support the idea that A. dufresnii presents high fenotipic plasticity that allows the species to have a wide distribution in different environments.












 uBio
uBio