Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.63 n.2 San José Apr./Jun. 2015
Functionality of arbuscular mycorrhizal fungi in three plant communities in the Managed Floristic Reserve San Ubaldo-Sabanalamar, Cuba
Funcionalidad de hongos micorrízicos arbusculares en tres comunidades vegetales de la Reserva Florística Manejada de San Ubaldo-Sabanalamar, Cuba
Funcionalidad de hongos micorrízicos arbusculares en tres comunidades vegetales de la Reserva Florística Manejada de San Ubaldo-Sabanalamar, Cuba
Eduardo Furrazola1*, Fernanda Covacevich2*, Yamir Torres-Arias1, Raquel M. Rodríguez-Rodríguez1, Juan F. Ley-Rivas1, Katiuska Izquierdo3*, Rigel Fernández-Valle4* & Ricardo Luis Louro Berbara5*
Abstract
Despite the ubiquity and importance of indigenous arbuscular mycorrhizal fungi (AMF) for plant ecosystems; functioning of indigenus mycorrhizal symbiosis (IMS) and related environmental factors at coastal Caribbean ecosystems remains still scarce. In order to determine functionality of IMS under contrasting land uses and wet seasons from Cuba, the influence of the water stress on some AMF functionality parameters from a semi-natural savannah (NS), a recovered savannah (RS) and an agro-ecosystem (AG) from the Managed Floristic Reserve San Ubaldo-Sabanalamar, Pinar del Rio, Cuba were assessed during two-years. Soil and root samples were collected in April and October, during the dry and wet seasons, respectively, in 2008 and 2010. Four plots in each ecosystem were selected, and five soil sub-samples were randomly collected, bulked, mixed homogeneously and used as the composite sample per plot. The host plant root biomass, arbuscular mycorrhizal colonization of the host plant, density of the intraradical and extraradical AMF mycelia, fungal endophyte biomass and AMF spore density were assessed. The host plant root biomass increased in the NS environment during the dry season, and approximately 12.85g root/dm3 dry soil was recorded. The colonization degree were significantly higher in all environments during the wet season of the second year, with means ranging from 79% to 89%. The extraradical mycelia were significantly more abundant in the dry season of the second year in all environments, with a maximum of 279mg/dm3 in the RS ecosystem. The density of AMF spores was highest in the dry season of the second year for the three studied ecosystems. The RS ecosystem hosted 5 670 spores/100g dry soil. In general, the influence of rainfall seasonality on the function of AMF was stronger than the influence of ecosystem management. The root biomass and extraradical mycelia were high in the dry seasons, suggesting strategies to increase the volume of soil for the mutual benefit of the symbionts. The increase in spore density during the dry seasons appears as an adaptation allowing AMF to survive period of water shortage. This study improves our understanding of the adaptative responses of arbuscular mycorrhizal symbiosis to seasonal variations in soil water availability. Rev. Biol. Trop. 63 (2): 341-356. Epub 2015 June 01.
Key words: mycorrhizae, ecosystems, savannas, dry and wet seasons.
Resumen
En el presente estudio se evaluó, durante dos años, la influencia de los períodos seco y lluvioso sobre el funcionamiento de hongos formadores de micorrizas arbusculares (HFMA) simbiontes de plantas nativas de una sabana semi natural, una sabana recuperada de la actividad agrícola y un agroecosistema, ubicados en la Reserva Florística Manejada San Ubaldo-Sabanalamar, Pinar del Río, Cuba. Se recolectaron muestras de suelo en abril y octubre (período seco y húmedo de 2008 y 2010, respectivamente). Dentro de cada ecosistema se seleccionaron cuatro parcelas y se recolectaron cinco submuestras al azar, las que fueron homogeneizadas para formar una muestra compuesta por parcela. Se cuantificó la biomasa de raicillas, la colonización micorrízica de las plantas hospedadoras en el campo, el micelio extrarradical, el micelio endófito y la densidad de esporas. La biomasa de raicillas en la sabana semi natural incrementó durante los períodos secos, la cual alcanzó hasta 12.85 g/dm3 de suelo. En todos los ecosistemas, la mayor colonización micorrízica ocurrió en la época lluviosa del segundo año con valores que oscilaron entre 79 y 89 %. Las mayores biomasas de micelio externo fueron registradas en los tres ecosistemas durante el período seco del segundo año, con un valor máximo de 279 mg/dm3 de suelo en la sabana recuperada. La mayor densidad de esporas de HFMA fue determinada en el periodo seco del segundo año para los tres ecosistemas estudiados, con el valor más alto en la sabana recuperada con 5 670 esporas/100 g de suelo seco. De manera general, se evidenció un efecto de la estacionalidad de la lluvia más que del manejo de ecosistema sobre el funcionamiento de los HFMA. La mayor actividad colonizadora se evidenció en períodos húmedos, mientras que mayor biomasa de raicillas y micelio externo durante los períodos secos, lo que sugiere estrategias de incremento del volumen del suelo explorado por parte de los HFMA como de las plantas hospedadoras. La proliferación de esporas evidenció la formación de estructuras de resistencia de los HFMA ante condiciones adversas. Los resultados obtenidos pondrían en evidencia la plasticidad de la simbiosis micorrízica ante variaciones en la disponibilidad de agua.
Palabras clave: hongos micorrízicos arbusculares, sabana, estaciones seca y lluviosa.
The ubiquity and importance of arbuscular mycorrhizal fungi (AMF) for plant-root symbiosis has been well documented for agricultural soils (Covacevich, Echeverría, & Aguirrezabal, 2007; Covacevich, & Echeverría, 2009; Oehl et al., 2009; Furrazola et al., 2011a; Herrera-Peraza, Hamel, Fernández, Ferrer, & Furrazola, 2011) and semi-natural ecosystems (Guadarrama, & Álvarez Sánchez, 1999; Lugo, & Cabello, 2002; Lovera, & Cuenca, 2007). Soil moisture (Anderson, & Dickman, 1984), organic matter and inorganic nutrient contents (Covacevich et al., 2007; Covacevich, Eyherabide, Sainz-Rozas, & Echeverría, 2012), and pH (Porter, Robson, & Abbott, 1987), among other soil factors, affect the distribution and functioning of AMF. In addition, seasonal water fluctuations also affect mycorrhizae dynamics (Guadarrama, & Álvarez Sánchez, 1999; Lugo & Cabello, 2002; Lugo, González-Maza, & Cabello, 2003). Many studies have been performed on the seasonal fungal community abundance patterns and, more specifically, on the variation of AMF formation of spores (Lugo, & Cabello, 2002; Cuenca, & Lovera, 2010; Becerra, Cabello, & Bartoloni, 2011). Furthermore, AMF spore production is associated with rainfall seasonality and host plant phenology (Sigüenza, Espejel, & Allen, 1996; Wilson, & Hartnett, 1997).
Although the majority of reports are from studies in Europe and the United States and, to a lesser extent, South America, information about the functioning of indigenous AMF from coastal Caribbean ecosystems remains scarce (Corkidi, & Rincón, 1997; Alarcón, & Cuenca, 2005). Although there are few reports regarding the activity and diversity of AMF in areas of the savannah of America, Lovera & Cuenca (2007) reported the high diversity of AMF in the Gran Sabana, Venezuela. The authors also indicated that both the infectivity and abundance of the Gigaspora and Scutellospora genus were susceptible to environmental disturbances. Furrazola et al. (2011b) also found a high AMF diversity in Cuba and reported a new species, Glomus crenatum, from Cuba. However, Turrini and Giovannetti (2011) stated that there is only limited information about AMF in protected areas from the Caribbean region. The San Ubaldo-Sabanalamar region, located in the Pinar del Río province of Cuba, belongs to the landscape Llanura Suroccidental of Pinar del Río. The geological emersion time, carbonated basement with karst process, combined with latitude, altitude, and W-E general disposition allow for differences in the solar light exposure, humidity, winds, and soil depth, which promote variable ecological conditions. As result of these conditions, a high diversity of ecotypes is found in this area, which determines speciation processes and a high plant endemism. By contrast, the threatened flora of the Pinar del Río province in Western Cuba ascend to 346 taxa, which represents approximately 10.55% from 3 278 vascular plants described in that province (Urquiola, González-Olia, Novo, & Acosta, 2010).
Rodríguez-Rodríguez (2013) and Rodríguez-Rodríguez, Herrera and Furrazola, (2013) assessed the rate of mycorrhizal colonization as an indicator of unique mycorrhizal activity. They evaluated the mycorrhizal colonization rate in five host species belonging to Asteraceae during the rainy and dry seasons at the Managed Floristic Reserve. However, other variables, such as AMF hyphae proliferation and/or spore production, may also contribute to a greater understanding of the activity of mycorrhizae and their function in relation to ecosystems and environmental variables; however, this information remains unknown. The objective of this study was to determine the function of indigenous mycorrhizal symbiosis of soils from the Managed Floristic Reserve San Ubaldo-Sabanalamar at Pinar del Río province in western Cuba under contrasting land uses and during two contrasting seasons, the dry and wet seasons, for a two year period.
Materials and methods
Study site: This study was performed in the Managed Floristic Reserve San Ubaldo-Sabanalamar, located in Pinar del Rio province, Western Cuba, the phytogeographic district of Sabana de Arenas Blancas (Samek, 1973) or Sabaloense (Borhidi, 1996). This reserve has 5 212ha that groups an ecotope uncommon in the country, with a high percent of endemics and endangered plants according to the International Union for the Conservation of Nature (IUCN). It is classified as a coastal plain fluvial-marine accumulative deltaic and lacustrine. The soils are Holocene sandy deposits that in Southwest and South are not related to the material that sustains it because they are formed by carbonated rocks from the Middle and High Miocene (Novo, Urquiola, & Ferro, 1984). The soils in the studied plots were a Haplic Arenosol, with a weighted average sandy loam texture according to the FAO (http://www.fao.org/docrep/011/a0510s/a0510s00.htm). In this reserve, the semi natural savannah is predominant, which is on quartzite white sands with secondary vegetal formations (Ricardo, Herrera, Cejas, Bastart, & Regalado, 2009).
The selected sites were as follows: i) a semi natural savannah (NS; 22°08’40’’4’’ N - 83°58’35’’2’’ W) with some degree of disturbance due to the low-intensive grazing of livestock; ii) a recovering savannah (RS; 22°09’14’’5’’ N - 83°57’41’’6’’ W), after eight years without any agricultural disruption, previously used as nursery areas for Nicotiana tabacum L. (snuff); and iii) an agroecosystem of low input (AG; 22°09’36’’1’’ N - 83°58’43’’5’’ W), used for subsistence farming continually for a minimum of ten years.
The semi natural savanna is dominated by (results of this study) Stylosanthes humilis Kunth, Cynodon dactylon (L.) Pers., Panicum erectifolium Nash, Sida brittonii León, Chamaecrista kunthiana (Schelcht., & Cham) H. S. Irwin & Barneby and Chamaecrista pygmaea (DC.) Britton var. savannarum (Britton) H.S. Irwin & Barneby during the wet period. During the dry season, the NS plant community is dominated by Scoparia dulcis L., Cynodon dactylon and Sida brittonii. In recovered savanna, Portulaca oleracea L., Panicum erectifolium Nash, Cyperus esculentus L., and Mimosa pudica L. are the predominant species during the wet period. During the dry period, the plant dominance changes to Panicum erectifolium, Rhynchelytrum repens (Willd.) Hubbard and Mecardonia procumbens (Mill.) Small. The agroecosystem soils are dominated by Eleusine indica (L.) Gaertner, Brachiaria distachya (L.) Stapf, Echinochloa colona (L.) Link., Digitaria ciliaris (Retz.) Koeler, Eryngium nasturtifolium Juss. and Heliotropium procumbens Mill., and the native crops are Musa paradisiaca L. (Banana), Zea mays L. (Maize) and Manihot esculenta Crantz. (Cassava), and Cucurbita pepo L. (Pumpkin).
Soil and plant sampling: The soil samples were collected on four days in April and October in 2008 and 2010. These sampling dates were selected to coincide with the end of the dry and wet periods, for April and October, respectively. For each sampling date and site, four replicate plots 1 000m2 (50×20m) were randomly set. Five soil cores (0.1×0.1m and 0.2m depth) were collected at each plot and homogenized into a composite sample, totaling approximately 1kg of collected soil per plot. After collection, the samples were stored in a cooler with ice packs (4°C in the dark for12h) to slow the microbial activity. In the laboratory, the samples were stored at 4°C in the dark for 24h until use. The samples were air-dried for 72h in darkness, sieved (2mm) and stored at room temperature (24-27°C).
For each of the twelve selected plots, five 0.25m2 quadrants were randomly established and plants within the quadrats were photographed and identified. The percentage of soil coverage by each plant species was assessed according to Yang, Hamel, Schellenberg, Perez and Berbara (2010).
Soil analysis: About 1kg of soil from each plot was used to determine the pH using a conductive glass electrode at a dilution of 1:2.5, organic matter content, using the Walkley-Black method according to Jackson (1982), exchangeable cations by extraction with 1 mol/L NH4 AC (pH 7) and determination by complexometric titration with EDTA for Ca and Mg and flame photometry for Na and K, and available P using extraction with 0.1 N H2SO4 by the method of Oniani according to INCA (1996). For gravimetric soil moisture (GSM), field-wet mass was measured. The samples were dried at 105°C for 48h and the GSM was calculated using the following equation: [(wet mass-dry mass)/(dry mass)×100] (Gardner, 1986). The bulk density (Blake & Hartge, 1986) was determined by collecting 5cm by 10.2cm cores, weighting the entire field-moist core, converting to a dry weight based on the GSM percentage, and dividing by the total volume of the soil in the core (200.2cm3).
Climate data: The white sandy areas of Western Pinar del Rio province belong to the thermoxerochimenic, mainly dry type climate, with three to four months of drought and annual rainfall between 1 200-1 400mm, according to the bioclimatic map of the New Atlas of Cuba (Vilamajó, 1989). The climatic data were obtained from a certified meteorological station (Isabel Rubio), and the information was used to assess the two contrasting seasons (dry and wet seasons) under study.
Arbuscular mycorrhizal soil components: The mycorrhizal components were determined according to Herrera-Peraza, Furrazola, Ferrer, Fernández-Valle and Torres-Arias (2004). Sequential soil sample sieving (from 140 to 40µm mesh) allowed for the elimination of all clay, loam and humus particles smaller than 40µm. The final sample was clean and consisted of primarily raw humus particles larger than 40µm, sand and gravel, rootlets, mycorrhizal components and other living organisms. The rootlet biomass was also determined in all soil samples and used to determine the endophyte biomass (see below).
AMF root colonization: Arbuscular mycorrhizal colonization (MC) was assessed by the gridline-intersect technique (Giovannetti, & Mosse, 1980) using a stereo microscope (CARL ZEISS-AXIOSKOP 2 Plus model) at ×150 magnification. Additionally, the intensity of AMF root colonization in each root segment was scored based on the presence of the fungus in the entire fragment using categories from zero to five (Herrera-Peraza et al., 2004). It was expressed as a percentage of visual density (VD%) and constitutes a modification of the Trouvelot, Kough and Gianinazzi-Pearson (1986) method, in which the number of intersections for each category (from 0 to 5) is then multiplied by 0.0, 1.0, 2.5, 15.5, 35.5, and 47.5%, which is the sum of all products divided by the total number of intersections.
Estimation of the endophyte biomass of AMF: The endophyte biomass of AMF (ED) in colonized mycorrhizal roots was estimated according to Herrera-Peraza et al. (2004) using following equation:
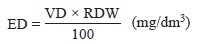
where RDW and VD represent the root dry weight and visual density of the mycorrhizae, respectively. A better approach to estimate ED was obtained by estimating the proportion (%) of cortical tissue in the root length instead of the total weight of the roots according to Herrera-Peraza et al. (2004). The weight of the epidermal tissue plus the rootlet central cylinder, which is usually 80% of the total root dry weight, was subtracted from the known dry weight of the roots.
Extraradical AMF mycelial biomass: The extraradical AMF mycelial biomass was determined according to Herrera-Peraza et al. (2004), based on the Giovannetti and Mosse (1980) method. Aliquots of approximately 40mg each dry soil sieved through 140 and 40µm, were spread with two or three drops of glycerol over a glass slide. Subsequently, a 22mm×22mm cover slip was placed carefully over the area containing the spread material to prevent the formation of air bubbles. Two aliquots were prepared for each fraction (140 and 40µm). All of the intersections of hyphae crossing four lines under the cover slip (two vertical and two horizontal) were counted using 100 to 200x magnifications with a compound microscope (CARL ZEISS model AXIOSKOP 2 Plus). The four lines were revised in length and depth such that the entire layer of material under the cover slip was scanned. The obtained mean value from the four lines was then multiplied by 0.000745, which yields the weight (mg) of the extraradical mycelium (EM) in each 40mg aliquot. The factor (0.000745) was estimated by multiplying the total number of lines in the 22×22 cover slip (20 lines) by the value of one intersection (1.57mm), and dividing the product by the length of the hyphae in 1mg EM (42 146.26mm) according to Herrera-Peraza et al. (2004). The extrapolation of the average weight of the EM from the two aliquots of each dry sieving soil fraction (140 µm and 40µm) gave the total EM/100g soil.
According to these authors, the accuracy of this method was verified by cutting polyester threads of 630mm length and spreading it under the cover slip. The result of three enumerations gave an average of 643±16mm, a variation coefficient of 2.57%, and an overestimation of 2.06%. The arbuscular mycorrhizal hyphae were not stained and recognized by color, diameter, angular projections and absence of septa according to Herrera-Peraza et al. (2004)
AMF spore isolation and counting: Spores from the field soils were extracted by wet sieving (Gerdemann, & Nicolson, 1963). Approximately 100g air-dried soils were suspended in 1L distilled water using a 2L beaker. The supernatant was decanted through sieves of 500, 140 and 40µm. The content of the 500µm sieve, primarily debris, was observed under the stereo microscope, to check for the presence of sporocarps. Aliquots of 10% or 5% of the dry weight of the 40 to 140µm sieved soil, respectively, were collected and centrifuged (2 000rpm for 5min). The sediment was resuspended in 60% sucrose solution and centrifuged (2 000rpm for one min). The spores in suspension were filtered and counted using a stereo microscope to extrapolate this number to 100g of soil. The morphological properties of the spores and their wall structure were observed by mounting the spores in polyvinyl alcohol/lactic acid/glycerol (PVLG), and a mixture of PVLG and Melzer’s reagent (1:1, v/v). The spores were microscopically examined and a brief taxonomic evaluation (mainly to a genus level) was conducted according to the http://invam.wvu.edu/the-fungi/species-descriptions.
The Kolmogorov-Smirnov test was used to ascertain the normality of the data, and the Levene test was used to check the homogeneity of variances. The data on the spore density were log(x+1) transformed and the AMF colonization and percentage of visual density were arcsin(x/100) transformed to meet the requirement of the analysis of variance (ANOVA). A two-way ANOVA was performed to test the significance of the factors periods (dry and wet) and ecosystems (NS, RS, AG). Principal component analysis was used to observe how the variables in this study interact in each ecosystem. To establish whether the spores produced were associated functionally with the extraradical mycelial biomass, a Pearson correlation between the AMF spore numbers in the soil and arbuscular extraradical mycelial biomass was also obtained. All statistical analyses were performed with INFOSTAT Version 2010 (Di Rienzo et al., 2010).
Results
Climatic conditions: The accumulated annual precipitation was 1 395.8 and 936.2mm for 2008 and 2010, respectively. The rainfall during the wet period of October 2008 was higher than normal, but lowers than normal in October 2010 (Fig. 1). During the dry periods of 2008 and 2010, the rainfalls were normal. The accumulated rainfall within three months before the end of each studied season were 167.1 and 117.7mm for the dry seasons of 2008 and 2010, and 861.5 and 448.2mm for the wet seasons of 2008 and 2010, respectively. The mean air temperature, which ranged from 20 to 25ºC, was historically normal for the dry (December-March) and wet (May-September) seasons.
Soils properties: The soil physical and chemical characteristics are shown in Table 1. The low organic matter and P contents, slightly acidic pH and low CEC recorded were typical for the studied ecosystem of quartzitic white sands. The more heavy soils (approximately 11% more dense), according to the bulk density, were found at the NS environment.
As expected, the soil moisture was higher for the wet seasons in the three studied ecosystems (Fig. 2) compared to the dry seasons; however, the differences among the ecosystems were more evident in 2008. The AG ecosystem recorded the highest soil moisture in the wet season of 2008.
Arbuscular mycorrhizal soil components: Root biomass: Although no significant interactions between the ecosystems and seasons were found for the rootlet biomass (Table 2), for a better understanding of the results, we analyzed the rootlet biomass for each year, season and environment. The root biomass decreased quantitatively from the NS to RS and AG environment (Fig. 3a). The highest root production was found during the dry period in the NS ecosystem, but no differences were found within seasons in the other environments.
AMF root colonization: Significant interactions between the ecosystems and seasons were obtained for the MC, and the individual effect of each parameter was also significant (Table 2). The MC was lower in 2008 than 2010 for all tested environments. In 2010, the MC was highest in the wet season at all tested environments (Fig. 3b).
Visual density of endophyte: Significant interactions between the ecosystems and seasons were obtained for the VD% (Table 2). Similar to the MC, the highest VD% was observed during the wet season in all environments during 2010, whereas the lowest VD% occurred in the wet season of 2008 (Fig. 3c). The highest VD% value was in the NS environment in the wet season of 2010, whereas the lowest values were found in the AG environment in the dry season of 2008.
Extraradical mycelial biomass: Significant interactions between the ecosystems and seasons were obtained for the extraradical mycelial biomass, and the individual effect of each parameter was also significant (Table 2). The extraradical mycelial biomass was highest in the dry season of all three environments in 2010 (Fig. 3d). In 2008, there were no differences in the extraradical mycelial biomass in the wet season.
Arbuscular endophyte biomass: Significant interactions occurred between the season and environment for the endophyte biomass (Table 2). The endophyte biomass was highest in the NS ecosystem, mainly in the dry season of 2008, and was three-fold higher than the biomass in the wet season. There was a clear decrease of endophyte production in the RS and AG environments in the dry season (Fig. 3e).
Extraradical mycelium endophyte biomass ratio: A significant interaction was found between the season and environment for the extraradical mycelium/endophyte biomass ratio and the individual effect of the ecosystem was significant (Table 2). The extraradical mycelium/endophyte biomass ratio increased from NS to AG (Fig. 3f). When differences among the seasons were significant, the highest extraradical mycelium:endophyte biomass ratio was recorded for the dry season at the RG and AG ecosystems.
AMF spore number and determination: There was a significant interaction between the season and environment for endophyte biomass (Table 2). In general, the highest spore density was found at both the NS and RS environments, mainly in the dry season of 2008, as well as in the AG environment of 2010 (Fig. 3g). Although significant, the relationship between the AMF extraradical mycelial biomass and the spore density was low (r=0.56, Pearson, p<0.05) and not explanatory (data not shown). At the NS environment, 20 AMF species were found, whereas 19 and 21 species were observed at the RS and AG environments, respectively (Table 3).
Ecosystem relationship based on analyzed mycorrhizal variables: Principal component analysis was performed on mycorrhizal variables for the two years evaluated, and showed that Axes 1 and 2 accounted for 49.1 and 33.3%, respectively, of the variability among the studied ecosystems (Fig. 4). In 2008, there was a tendency for the season (dry and wet) to cluster together with the year for all tested parameters, explaining the AMF functioning. In the environment RS, a certain degree of clustering occurred in the rainy season, and the AG environment showed clustering with the extraradical mycelium:endophyte biomass ratio (Fig. 4).
Discussion
The soil physico-chemical properties for the late April and October sampling dates were similar for both periods. Soils were characterized by low mineral content, low cation exchange capacity and low levels of organic matter, which are typical of the quartzitic sandy soils of the Pinar del Rio province. Similar results were obtained by Urquiola et al. (2010) and Villate, Herrera, Urquiola and Ricardo (2010). The soil moisture was higher in April than in October of 2010, following the normal pattern of seasonal variation in the Pinar del Rio province, where a cool early summer characterized by soil moisture sufficiency is followed by a warm and dry period. However, during the first sampling of the rainy season (year 2008), unusually high soil moisture content was found in relation to the dry season of the same year and the wet season of 2010. This may be due to flooding in the area caused by the hurricanes Gustav and Ike in September of that year, which had a cumulative average monthly rainfall of 483mm.
Although the results were not consistent for all ecosystems, seasons and years, a general trend towards greater development of rootlets biomass in the dry periods (mainly at the less disturbed ecosystem (NS)) was recorded. This is consistent with results by Hernández and Fiala (1992) for a savannah dominated by Paspalum notatum Flügge and by Hernández, Rodríguez, Crespo, Sandrino and Fraga (2011) for various grasslands at Mayabeque province (Cuba). The highest production of small roots during the dry period may be explained by the adaptive response of certain plants that develop deeper root systems to access water in the dry season (Nepstad et al., 1994). Studies by Hernández & Sánchez (2012) found that soil moisture plays an important role in the production of fine roots in forest systems. It is known that sandy soils have low water storage capacity. In our study, the proportion of sand in the soils was high and soil moisture may have been low, particularly in the dry season. Plants obtain more water by allocating more energy to root development, which could contribute to promote plant diversity. In our study, this phenomenon was more evident at the NS ecosystem, where plant diversity was higher than in the RS and AG ecosystems.
The estimation of mycorrhizal colonization is a useful parameter of rapid evaluation because it shows the correlation of the growth responsiveness of AMF to all treatments combinations (Covacevich, & Echeverria, 2010). Despite some inconsistencies in the evaluated ecosystems, seasons or years, in general, greater root mycorrhizal colonization was observed in the wet periods, mainly 2010. This trend is also valid for April of 2008, when the highest values of mycorrhizal colonization were obtained, was a wetter month than traditionally observed, as the values of rainfall for January, March and April of 2008 averaged 56mm as opposed to 39mm average for the same period in 2010 and 45mm average for the last 10 years in that region.
Apple et al. (2005) and Cuenca and Lovera (2010) also reported higher mycorrhizal colonization of plant roots in the rainy period in other tropical and desert environments. Most seasonal studies focus on representative plant species rather than the entire plant community as indicated by Mandyam & Jumpponen (2008). In this respect, Sanders and Fitter (1992) suggested that it is essential to follow the seasonal dynamics of AMF in entire plant communities and emphasize that true seasonal patterns can be observed when colonization levels are followed for more than a year. The results of our study were obtained from roots of the entire plant communities from three contrasting ecosystems. Therefore, the mycorrhizal activity parameters obtained may be representative of the AMF host community of the ecosystems, and not only associated with a host species. DeMars and Boerner (1995) found seasonal variations in mycorrhizal development in some herbaceous species and suggested that plants with long root life cycles typically exhibit persistent mycorrhizal colonization that varies seasonally, as observed in our study. The high colonization rates found in our study (over 50% for all sampling dates) are consistent with the report of Read, Koucheki and Hodgson (1976). In environments where competitive situations prevail, species are strongly mycorrhizal and values of mycorrhizal colonization are usually higher than 50%.
The lowest values of AMF root colonization in the three ecosystems during the first rainy season (year 2008) may be due to flooding in the area caused by unusual rain. It is known that the study area is prone to seasonal flooding, which was severe in 2008. Typically, decreased levels of oxygen in soils are attributed to high soil water content. This may adversely affect root growth and the growth of aerobic microorganisms, such as AMF (Miller, 2000; Bohrer, Friese, & Amon, 2004). Consequently, the low root colonization level found during the wet season (2008 year) in our study is unusual. Anaerobic conditions created by flooding may explain the unexpectedly low levels of mycorrhizal colonization that we observed during the rainy season of 2008.With lower soil moisture content (year 2010), the highest levels of mycorrhizal colonization were recorded during the wet season. Therefore, we hypothesize that normal water content in the soil favors mycorrhizal symbiosis formation.
Adaptation to environmental conditions may occur within an AM fungal community or within a mycelium, and alternatively, different temporal pattern of sporulation of AMF may be found, as reported by Yang et al. (2010). The higher extraradical mycelium biomass and the high AMF sporulation recorded in the dry period of 2010, may be strategies to obtain water and develop resistant structures to survive adverse conditions. Similar trends have been reported by Lugo, Anton and Cabello (2005) and Oehl et al. (2009). In addition, Fitter (1987) established that under growth conditions characterized by moisture deficits (in our study low precipitation occurred from September to October in 2010, which was significantly lower than historical values), the cost to produce rootlets is greater than the cost to produce or sustain extraradical hyphae. This may explain the abundance of mycelium in all three ecosystems during the dry season. The highest density of AMF spores observed in the dry period in our study is consistent with other reports (Guadarrama, & Álvarez-Sánchez, 1999; Lugo, & Cabello, 2002; Cuenca, & Lovera, 2010). Bever (1994) also found that in tropical ecosystems, rains favor AMF spores germination, thereby decreasing their presence in soil during wetter seasons. The results of the brief taxonomic determination indicate no differences between AMF diversity among the tested environments. Future studies should further expand taxonomic determinations to the species level.
Our measurements of AM endophyte biomass correspond to measurements made by others. Morales (1995) quantified endophyte biomass between 7 and 198mg/dm3 of soil in some agroecosystems with traditional management, but in different successional stages in the paramo of Gavidia, Venezuela. Herrera-Peraza & Furrazola (2003) reported values between 57 and 91mg/dm3 of soil in six different successional plots at the evergreen forest located in the Biosphere Reserve Sierra del Rosario, Cuba, whereas Furrazola (2003) observed values up to 97mg/dm3 of soil in different Venezuelan and Cuban ecosystems belonging to the paramos, the savannas and the evergreen forest in both countries.
Herrera, Rodríguez, Orozco, Furrazola and Ferrer (1988) identified the relationship between extraradical mycelial and endophyte biomasses as a measure of the mutualistic level of AM symbiosis. Values greater than unity suggest a parasitic phase of symbiosis and the likelihood of nutrient excess or nutritional imbalances in the substrate (Bowen, 1985), poor light or unfavorable soil pH (Herrera et al. 1988). The highest extraradical mycelial biomass:endophyte biomass ratio of AMF in the AG ecosystem, regardless of the season analyzed, supports our hypothesis that there is a disruptive effect of this human activity on the arbuscular mycorrhizal symbiosis. We conclude that agricultural activities, such as tillage, erosion, high levels of nutrients (particularly P) and frequent fallow periods decrease the abundance of AMF propagules, such as spores and infective mycelium (Karasawa, & Takebe, 2011; Schnoor, Lekberg, Rosendahl, & Olsson, 2011). Furthermore, it has been proposed by Johnson, Graham and Smith (1997) that mycorrhizal associations could be considered symbioses that functionally range along a continuum of parasitism to mutualism and that environmental conditions, particularly the abundance of soil nutrients, could determine the position of AMF along that continuum. The highest values of this relationship during the drier season of 2010 could be a response to an extremely dry environment, which may inhibit the expression of a mutualistic relationship as reported by Yang et al. (2010).
In 2010, the dry period increased the extraradical mycelial biomass/endophyte biomass ratio, and spore density, as reported earlier by Oliveira (2001) and He, Mouratov and Steinberger (2002). Higher soil moisture content during the rainy season increased the percentages of mycorrhizal root colonization, visual density and arbuscular mycorrhizal endophyte biomass in 2010 in the three ecosystems.
In conclusion, we report for the first time the seasonality of root colonization, production of soil extraradical mycelial biomass and spores by AMF in this type of ecosystems. A high number of endemic and endangered plants were studied in this experiment, but much has yet to be learned about the nature of mycorrhizal function at the ecosystem level. The results of this study indicate that the function of AMF indigenous to the Managed Floristic Reserve San Ubaldo-Sabanalamar (Pinar del Rio, Cuba) varies depending on environmental factors, particularly the timing of rainfall events and soil moisture.
Acknowledgments
We thank G. Hernández and N. E. Ricardo Nápoles for their valuables comments on the manuscript, R. Osbel Gómez for technical assistance and L. Hernández, conservation’s worker, of RFM San Ubaldo-Sabanalamar for its valuable help in field expeditions. We thank also J. A. Sánchez Rendón for his useful comments related to statistical analyses. We are grateful to Chantal Hamel also for their helpful comments. We are also thankful to CNPq (Universal and Research Productivity) and Faperj (APQ1) to the grants given to R. L. L. Berbara.
References
Anderson, R. C., & Dickman, L. A. (1984). Interaction of vascular plants and vesicular-arbuscular mycorrhizal fungus across a soil moisture-nutrient gradient. Oecologia, 64, 111-117. [ Links ]
Alarcón, C., & Cuenca, G. (2005). Arbuscular mycorrhizas in coastal sand dunes of the Paraguaná Peninsula, Venezuela. Mycorrhiza, 16, 1-9. [ Links ]
Apple, M. E., Thee, C. I., Smith-Logonzo, V. L., Cogar, C. R., Wells, C. E., & Nowak, R. S. (2005). Arbuscular mycorrhizal colonization of Larrea tridentata and Ambrosia dumosa roots varies with precipitation and season in the Mojave Desert. Symbiosis, 39, 131-135. [ Links ]
Becerra, A. G., Cabello, M. N., & Bartoloni, N. J. (2011). Native arbuscular mycorrhizal fungi in the Yungas forests, Argentina. Mycologia, 103(2), 273-279. [ Links ]
Bever, J. D. (1994). Feedback between plants and their soil communities in an old-field community. Ecology, 75, 1965-1978. [ Links ]
Blake, G. R., & Hartge, K. H. (1986). Bulk density. In A. Klute (Ed.), Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods (No. 9, 2nd ed., pp. 363-375). Madison: Agronomy Monograph. [ Links ]
Bohrer, K. E., Friese, C. F., & Amon, J. P. (2004). Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats. Mycorrhiza, 14, 329-337. [ Links ]
Borhidi, A. (1996). Phytogeography and Vegetation Ecology of Cuba. Budapest: Akademiai Kiado. [ Links ]
Bowen, G. D. (1985). The rate of assimilates and the cost of vesicular-arbuscular mycorrhizal symbiosis (Technical document). Vienna, Austria: International Atomic Energy Agency. [ Links ]
Corkidi, L., & Rincón, E. 1997. Arbuscular mycorrhizae in a tropical sand dune ecosystem on the Gulf of Mexico. I. Mycorrhizal status and inoculum potential along a successional gradient. Mycorrhiza, 7, 9-15. [ Links ]
Covacevich, F., Echeverría, H. E., & Aguirrezabal, L. A. N. (2007). Soil available phosphorus status determines indigenous mycorrhizal colonization into field and glasshouse-grown spring wheat in Argentina. Applied Soil Ecology, 35, 1-9. [ Links ]
Covacevich, F., & Echeverría, H. E. (2009). Mycorrhizal occurrence and responsiveness in tall fescue and wheatgrass are affected by the source of phosphorus fertilizer and fungal inoculation. Journal of Plant Interactions, 4(2), 101-112. [ Links ]
Covacevich, F., & Echeverría, H. E. (2010). Indicadores para seleccionar inóculos de hongos micorricicos arbusculares eficientes en suelos moderadamente ácidos. Ciencia del Suelo, 28(1), 9-22. [ Links ]
Covacevich, F., Eyherabide, M., Sainz-Rozas, H. R., & Echeverría, H. E. (2012). Capacidad micotrófica arbuscular y características químicas de suelos agrícolas y prístinos de Buenos Aires (Argentina). Ciencia del Suelo, 30(2), 119-128. [ Links ]
Cuenca, G., & Lovera, M. (2010). Seasonal variation and distribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical Sclerophyllous shrubland. Botany, 88, 54-64. [ Links ]
DeMars, B. G., & Boerner, R. E. J. (1995). Mycorrhizal dynamics of three woodland herbs of contrasting phenology along topographic gradients. American Journal of Botany, 82, 1426-1431. [ Links ]
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada M., & Robledo, C. W. (2010). InfoStat, software Estadística, v. 2011. Universidad Nacional de Córdoba, Facultad de Ciencias Agrarias: Grupo InfoStat. [ Links ]
Fitter, A. H. (1987). An architectural approach to the comparative ecology of plant root systems. New Phytologist, 106, 61-67. [ Links ]
Furrazola, E. (2003). Tendencias funcionales de las micorrizas arbusculares en ecosistemas naturales y de reemplazo de Cuba y Venezuela (Tesis de Maestría). Cuba: Instituto de Ecología y Sistemática. [ Links ]
Furrazola, E., Ferrer, R. L., Orozco, M. O., Torres-Arias, Y., Collazo, E., & Herrera-Peraza, R. A. (2011a). Especies de hongos micorrizógenos arbusculares (Glomeromycota) en un agroecosistema de la provincia La Habana, con un nuevo reporte para Cuba, Glomus glomerulatum. Acta Botánica Cubana, 210, 26-30. [ Links ]
Furrazola, E., Torres-Arias, Y., Ferrer, R. L., Herrera, R. A., Berbara, R. L. L., & Goto, B. T. (2011b). Glomus crenatum (Glomeromycetes), a new ornamented species from Cuba. Mycotaxon, 116, 143-149. [ Links ]
Gardner, W. H. (1986). Water content. In A. Klute (Ed.), Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods (pp. 516-517). Madison, Wisconsin, USA: Soil Science Society of America. [ Links ]
Gerdemann, J. W., & Nicolson, T. J. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions British. Mycological Society, 46, 235-244. [ Links ]
Giovannetti, M., & Mosse, B. (1980). An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist, 84(3), 489-500. [ Links ]
Guadarrama, P., & Álvarez-Sánchez, F. J. (1999). Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza, 8(5), 267-270. [ Links ]
He, X., Mouratov, S., & Steinberger, Y. (2002). Temporal and spatial dynamics of vesicular-arbuscular mycorrhizal fungi under the canopy of Zygophyllum dumosum Boiss. in the Negev Desert. Journal of Arid Environments, 5, 379-387. [ Links ]
Hernández, L., & Fiala, K. (1992). Root biomass dynamics in the savanna community of Paspaletum notati in Cuba. Ekológia CSFR, 11(2), 153-166. [ Links ]
Hernández, L., Rodríguez, I., Crespo, G., Sandrino, B., & Fraga, S. (2011). Componentes de la fitomasa en siete pastizales de La Habana, Cuba. Acta Botánica Cubana, 212, 27-34. [ Links ]
Hernández, L., & Sánchez, J. A. (2012). Dinámica de la humedad del suelo y la fitomasa de raíces en ecosistemas de la Sierra del Rosario, Cuba. Pastos y Forrajes, 35, 79-98. [ Links ]
Herrera, R. A., Rodríguez, M. E., Orozco, M. O., Furrazola, E., & Ferrer, R. L. (1988). Las micorrizas y el funcionamiento de los bosques tropicales (Chap. 28). In R. A. Herrera, L. Menéndez, M. E. Rodríguez, & E. E. García (Eds.), Ecología de los bosques siempreverdes de la Sierra del Rosario, Cuba (pp. 627-670). Uruguay: ROSTLAC, Proyecto MAB No. 1, 1974-1987. [ Links ]
Herrera-Peraza, R. A., & Furrazola, E. (2003). Influência das taxas de renovação da necromassa no funcionamento exuberante ou austero de micorrizas vesicular-arbusculares (MVA) em floresta tropical. In P. Yoshio Kageyama, R. E. de Oliveira, L. F. Duarte de Moraes, & F. B. Gandara (Eds.), Restauração Ecológica de Ecossistemas Naturais (pp. 167-184). Sao Paulo, Brasil: Livraria e Editora Agropecuaria. [ Links ]
Herrera-Peraza, R. A., Furrazola, E., Ferrer, R. L., Fernández-Valle, R., & Torres-Arias, Y. (2004). Functional strategies of root hairs and arbuscular mycorrhizae in an evergreen tropical forest, Sierra del Rosario, Cuba. Revista CENIC Ciencias Biológicas, 35(2), 113-123. [ Links ]
Herrera-Peraza, R. A., Hamel, C., Fernández, F., Ferrer, R. L., & Furrazola, E. (2011). Soilstrain compatibility: the key to effective use of arbuscular mycorrhizal inoculants? Mycorrhiza, 21, 183-193. [ Links ]
Instituto Nacional de Ciencias Agrícolas (INCA). (1996). Manual de técnicas analíticas del laboratorio de Agroquímica. La Habana, Cuba: Instituto Nacional de Ciencias Agrícolas. [ Links ]
Jackson, M. L. (1982). Análisis químico de suelo(4ta ed.). Barcelona, España: Ediciones Omega. [ Links ]
Johnson, N. C., Graham, J. H., & Smith, F. A. (1997). Functioning of mycorrhizal associations along the mutualism parasitism continuum. New Phytologist, 135, 575-586. [ Links ]
Karasawa, T., & Takebe, M. (2011). Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant and Soil, 353, 355-366. [ Links ]
Lovera, M., & Cuenca, G. (2007). Diversidad de hongos micorrízicos arbusculares (HMA) y potencial micorrízico del suelo de una sabana natural y una sabana perturbada de La Gran Sabana, Venezuela. Interciencia, 32(2), 108-114. [ Links ]
Lugo, M. A., & Cabello, M. N. (2002). Native arbuscular mycorrhizal fungi (AMF) from mountain grassland (Córdoba, Argentina) I. Seasonal variation of fungal spore diversity. Mycologia, 94(4), 579-586. [ Links ]
Lugo, M. A., González-Maza, M. E., & Cabello, M. N. (2003). Arbuscular mycorrhizal fungi in a mountain grassland II: Seasonal variation of colonization studied, along with its relation to grazing and metabolic host type. Mycologia, 95(3), 407-415. [ Links ]
Lugo, M. A., Anton, A. M., & Cabello, M. N. (2005). Arbuscular mycorrhizas in the Larrea divaricata scrubland of the arid ‘Chaco’, Central Argentina. Journal of Agricultural Technology, 1, 163-178. [ Links ]
Mandyam, K., & Jumpponen, A. (2008). Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza, 18, 145-155. [ Links ]
Miller, S. P. (2000). Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrological gradient. New Phytologist, 145, 145-155. [ Links ]
Morales, S. (1995). Actividad micorrízica y diversidad de Glomales en parcelas de diferentes estadios sucesionales en el páramo de Gavidia, Venezuela (Tesis de Licenciatura). Universidad de los Andes, Mérida, Venezuela. [ Links ]
Nepstad, D. C., de Carvalho, C. R., Davidson, E. A., Jipp, P. H., Lefebvre, P. A., & Negreiros, G. H. (1994). The role of deep roots in the hydrological and carbon cycles of amazonian forests and pastures. Nature, 372, 666-669. [ Links ]
Novo, R., Urquiola, A. J., & Ferro, J. (1984). Contribución al estudio de los ecosistemas de arenas blancas. IV Reunión Científica de Profesores del Instituto Superior Pedagógico de Pinar del Río, Cuba. [ Links ]
Oehl, F., Sieverding, E., Ineichen, K., Mäder, P., Wiemken, A., & Boller, T. (2009). Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agriculture Ecosystems and Environment, 134, 257-268. [ Links ]
Oliveira, A. N. (2001). Fungos micorrízicos arbusculares e teores de nutrientes em plantas de cupuaçu e guaraná de um Sistema Agroflorestal na região de Manaus, AM (Master dissertation). Universidade Federal do Amazonas, Brasil. [ Links ]
Porter, W. M., Robson, A. D., & Abbott, L. K. (1987). Field survey of the distribution of VAM fungi in relation to soil pH. Journal of Applied Ecology, 24, 659-662. [ Links ]
Read, D. J., Koucheki, H. K., & Hodgson, J. (1976). Vesicular-arbuscular mycorrhiza in natural vegetation systems. I-The occurrence of infection. New Phytologist, 77, 641-653. [ Links ]
Ricardo, N., Herrera, P. P., Cejas, F., Bastart, J. A., & Regalado, T. (2009). Tipos y características de las formaciones vegetales de Cuba. Acta Botánica Cubana, 203, 1-42. [ Links ]
Rodríguez-Rodríguez, R. M. (2013). Potencialidades de uso de las micorrizas arbusculares en la conservación de plantas cubanas. Bissea, 7(3). [ Links ]
Rodríguez-Rodríguez, R. M., Herrera, P., & Furrazola, E. (2013). Arbuscular mycorrhizal colonization in Asteraceae from white sand savannas, in Pinar del Río, Cuba. Biota Neotropica, 13(3), 136-140. [ Links ]
Samek, V. (1973). Regiones fitogeográfitas de Cuba. Academia de Ciencias de Cuba. Serie Forestal, 15, 1-63. [ Links ]
Sanders, I. R. & Fitter, A. H. (1992). The ecology and functioning of vesicular-arbuscular mycorrhizas in co-existing grassland species I. Seasonal patterns of mycorrhizal occurrence and morphology. New Phytologist, 120, 517-524. [ Links ]
Schnoor, T. K., Lekberg, Y., Rosendahl, S., & Olsson, P. A. (2011). Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in natural grassland. Mycorrhiza, 21, 211-220. [ Links ]
Sigüenza, C, Espejel, I., & Allen, E. B. (1996). Seasonality of mycorrhizae in coastal sand dunes of Baja California. Mycorrhiza, 6, 151-157. [ Links ]
Trouvelot, A, Kough, J. L., & Gianinazzi-Pearson, V. (1986). Mesure du taux de mycorrhization VA d’un syste`me radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionelle. In V. Gianinazzi-Pearson, & S. Gianinazzi (Eds.), Physiological and Genetical Aspects of mycorrhiza (pp. 101-109). Paris: INRA. [ Links ]
Turrini, A., & Giovannetti, M. (2011). Arbuscular mycorrhizal fungi in national parks, nature reserves and protected areas worldwide: a strategic perspective for their in situ conservation. Mycorrhiza, 22, 81-97. [ Links ]
Urquiola, A. J., González-Olia, L., Novo, R., & Acosta, Z. (2010). Libro rojo de la flora vascular de la provincia de Pinar del Río. Cuba, Jardín Botánico de Pinar del Río: Publicaciones Universidad de Alicante. [ Links ]
Vilamajó, D. (1989). Mapa de Bioclima. La Habana, Cuba: Nuevo Atlas Nacional de Cuba. [ Links ]
Villate, M., Herrera, P. P., Urquiola, A. J., & Ricardo, N. E. (2010). Flora sinántropa en las comunidades terrestres de la Reserva Florística Manejada San Ubaldo-Sabanalamar, Pinar del Río, Cuba. Acta Botánica Cubana, 207, 35-44. [ Links ]
Wilson, G. T. W., & Hartnett, D. C. (1997). Effects of mycorrhizae on plant growth and dynamics in experimental tallgrass prairie microcosms. American Journal of Botany, 84, 478-482. [ Links ]
Yang, C., Hamel, C., Schellenberg, M. P., Perez, J. C., & Berbara, R. L. L. (2010). Diversity and functionality of arbuscular mycorrhizal fungi in three plant communities in semiarid grasslands National Park, Canada. Microbial Ecology, 59, 724-733. [ Links ]
Alarcón, C., & Cuenca, G. (2005). Arbuscular mycorrhizas in coastal sand dunes of the Paraguaná Peninsula, Venezuela. Mycorrhiza, 16, 1-9. [ Links ]
Apple, M. E., Thee, C. I., Smith-Logonzo, V. L., Cogar, C. R., Wells, C. E., & Nowak, R. S. (2005). Arbuscular mycorrhizal colonization of Larrea tridentata and Ambrosia dumosa roots varies with precipitation and season in the Mojave Desert. Symbiosis, 39, 131-135. [ Links ]
Becerra, A. G., Cabello, M. N., & Bartoloni, N. J. (2011). Native arbuscular mycorrhizal fungi in the Yungas forests, Argentina. Mycologia, 103(2), 273-279. [ Links ]
Bever, J. D. (1994). Feedback between plants and their soil communities in an old-field community. Ecology, 75, 1965-1978. [ Links ]
Blake, G. R., & Hartge, K. H. (1986). Bulk density. In A. Klute (Ed.), Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods (No. 9, 2nd ed., pp. 363-375). Madison: Agronomy Monograph. [ Links ]
Bohrer, K. E., Friese, C. F., & Amon, J. P. (2004). Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats. Mycorrhiza, 14, 329-337. [ Links ]
Borhidi, A. (1996). Phytogeography and Vegetation Ecology of Cuba. Budapest: Akademiai Kiado. [ Links ]
Bowen, G. D. (1985). The rate of assimilates and the cost of vesicular-arbuscular mycorrhizal symbiosis (Technical document). Vienna, Austria: International Atomic Energy Agency. [ Links ]
Corkidi, L., & Rincón, E. 1997. Arbuscular mycorrhizae in a tropical sand dune ecosystem on the Gulf of Mexico. I. Mycorrhizal status and inoculum potential along a successional gradient. Mycorrhiza, 7, 9-15. [ Links ]
Covacevich, F., Echeverría, H. E., & Aguirrezabal, L. A. N. (2007). Soil available phosphorus status determines indigenous mycorrhizal colonization into field and glasshouse-grown spring wheat in Argentina. Applied Soil Ecology, 35, 1-9. [ Links ]
Covacevich, F., & Echeverría, H. E. (2009). Mycorrhizal occurrence and responsiveness in tall fescue and wheatgrass are affected by the source of phosphorus fertilizer and fungal inoculation. Journal of Plant Interactions, 4(2), 101-112. [ Links ]
Covacevich, F., & Echeverría, H. E. (2010). Indicadores para seleccionar inóculos de hongos micorricicos arbusculares eficientes en suelos moderadamente ácidos. Ciencia del Suelo, 28(1), 9-22. [ Links ]
Covacevich, F., Eyherabide, M., Sainz-Rozas, H. R., & Echeverría, H. E. (2012). Capacidad micotrófica arbuscular y características químicas de suelos agrícolas y prístinos de Buenos Aires (Argentina). Ciencia del Suelo, 30(2), 119-128. [ Links ]
Cuenca, G., & Lovera, M. (2010). Seasonal variation and distribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical Sclerophyllous shrubland. Botany, 88, 54-64. [ Links ]
DeMars, B. G., & Boerner, R. E. J. (1995). Mycorrhizal dynamics of three woodland herbs of contrasting phenology along topographic gradients. American Journal of Botany, 82, 1426-1431. [ Links ]
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada M., & Robledo, C. W. (2010). InfoStat, software Estadística, v. 2011. Universidad Nacional de Córdoba, Facultad de Ciencias Agrarias: Grupo InfoStat. [ Links ]
Fitter, A. H. (1987). An architectural approach to the comparative ecology of plant root systems. New Phytologist, 106, 61-67. [ Links ]
Furrazola, E. (2003). Tendencias funcionales de las micorrizas arbusculares en ecosistemas naturales y de reemplazo de Cuba y Venezuela (Tesis de Maestría). Cuba: Instituto de Ecología y Sistemática. [ Links ]
Furrazola, E., Ferrer, R. L., Orozco, M. O., Torres-Arias, Y., Collazo, E., & Herrera-Peraza, R. A. (2011a). Especies de hongos micorrizógenos arbusculares (Glomeromycota) en un agroecosistema de la provincia La Habana, con un nuevo reporte para Cuba, Glomus glomerulatum. Acta Botánica Cubana, 210, 26-30. [ Links ]
Furrazola, E., Torres-Arias, Y., Ferrer, R. L., Herrera, R. A., Berbara, R. L. L., & Goto, B. T. (2011b). Glomus crenatum (Glomeromycetes), a new ornamented species from Cuba. Mycotaxon, 116, 143-149. [ Links ]
Gardner, W. H. (1986). Water content. In A. Klute (Ed.), Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods (pp. 516-517). Madison, Wisconsin, USA: Soil Science Society of America. [ Links ]
Gerdemann, J. W., & Nicolson, T. J. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions British. Mycological Society, 46, 235-244. [ Links ]
Giovannetti, M., & Mosse, B. (1980). An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist, 84(3), 489-500. [ Links ]
Guadarrama, P., & Álvarez-Sánchez, F. J. (1999). Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza, 8(5), 267-270. [ Links ]
He, X., Mouratov, S., & Steinberger, Y. (2002). Temporal and spatial dynamics of vesicular-arbuscular mycorrhizal fungi under the canopy of Zygophyllum dumosum Boiss. in the Negev Desert. Journal of Arid Environments, 5, 379-387. [ Links ]
Hernández, L., & Fiala, K. (1992). Root biomass dynamics in the savanna community of Paspaletum notati in Cuba. Ekológia CSFR, 11(2), 153-166. [ Links ]
Hernández, L., Rodríguez, I., Crespo, G., Sandrino, B., & Fraga, S. (2011). Componentes de la fitomasa en siete pastizales de La Habana, Cuba. Acta Botánica Cubana, 212, 27-34. [ Links ]
Hernández, L., & Sánchez, J. A. (2012). Dinámica de la humedad del suelo y la fitomasa de raíces en ecosistemas de la Sierra del Rosario, Cuba. Pastos y Forrajes, 35, 79-98. [ Links ]
Herrera, R. A., Rodríguez, M. E., Orozco, M. O., Furrazola, E., & Ferrer, R. L. (1988). Las micorrizas y el funcionamiento de los bosques tropicales (Chap. 28). In R. A. Herrera, L. Menéndez, M. E. Rodríguez, & E. E. García (Eds.), Ecología de los bosques siempreverdes de la Sierra del Rosario, Cuba (pp. 627-670). Uruguay: ROSTLAC, Proyecto MAB No. 1, 1974-1987. [ Links ]
Herrera-Peraza, R. A., & Furrazola, E. (2003). Influência das taxas de renovação da necromassa no funcionamento exuberante ou austero de micorrizas vesicular-arbusculares (MVA) em floresta tropical. In P. Yoshio Kageyama, R. E. de Oliveira, L. F. Duarte de Moraes, & F. B. Gandara (Eds.), Restauração Ecológica de Ecossistemas Naturais (pp. 167-184). Sao Paulo, Brasil: Livraria e Editora Agropecuaria. [ Links ]
Herrera-Peraza, R. A., Furrazola, E., Ferrer, R. L., Fernández-Valle, R., & Torres-Arias, Y. (2004). Functional strategies of root hairs and arbuscular mycorrhizae in an evergreen tropical forest, Sierra del Rosario, Cuba. Revista CENIC Ciencias Biológicas, 35(2), 113-123. [ Links ]
Herrera-Peraza, R. A., Hamel, C., Fernández, F., Ferrer, R. L., & Furrazola, E. (2011). Soilstrain compatibility: the key to effective use of arbuscular mycorrhizal inoculants? Mycorrhiza, 21, 183-193. [ Links ]
Instituto Nacional de Ciencias Agrícolas (INCA). (1996). Manual de técnicas analíticas del laboratorio de Agroquímica. La Habana, Cuba: Instituto Nacional de Ciencias Agrícolas. [ Links ]
Jackson, M. L. (1982). Análisis químico de suelo(4ta ed.). Barcelona, España: Ediciones Omega. [ Links ]
Johnson, N. C., Graham, J. H., & Smith, F. A. (1997). Functioning of mycorrhizal associations along the mutualism parasitism continuum. New Phytologist, 135, 575-586. [ Links ]
Karasawa, T., & Takebe, M. (2011). Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant and Soil, 353, 355-366. [ Links ]
Lovera, M., & Cuenca, G. (2007). Diversidad de hongos micorrízicos arbusculares (HMA) y potencial micorrízico del suelo de una sabana natural y una sabana perturbada de La Gran Sabana, Venezuela. Interciencia, 32(2), 108-114. [ Links ]
Lugo, M. A., & Cabello, M. N. (2002). Native arbuscular mycorrhizal fungi (AMF) from mountain grassland (Córdoba, Argentina) I. Seasonal variation of fungal spore diversity. Mycologia, 94(4), 579-586. [ Links ]
Lugo, M. A., González-Maza, M. E., & Cabello, M. N. (2003). Arbuscular mycorrhizal fungi in a mountain grassland II: Seasonal variation of colonization studied, along with its relation to grazing and metabolic host type. Mycologia, 95(3), 407-415. [ Links ]
Lugo, M. A., Anton, A. M., & Cabello, M. N. (2005). Arbuscular mycorrhizas in the Larrea divaricata scrubland of the arid ‘Chaco’, Central Argentina. Journal of Agricultural Technology, 1, 163-178. [ Links ]
Mandyam, K., & Jumpponen, A. (2008). Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza, 18, 145-155. [ Links ]
Miller, S. P. (2000). Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrological gradient. New Phytologist, 145, 145-155. [ Links ]
Morales, S. (1995). Actividad micorrízica y diversidad de Glomales en parcelas de diferentes estadios sucesionales en el páramo de Gavidia, Venezuela (Tesis de Licenciatura). Universidad de los Andes, Mérida, Venezuela. [ Links ]
Nepstad, D. C., de Carvalho, C. R., Davidson, E. A., Jipp, P. H., Lefebvre, P. A., & Negreiros, G. H. (1994). The role of deep roots in the hydrological and carbon cycles of amazonian forests and pastures. Nature, 372, 666-669. [ Links ]
Novo, R., Urquiola, A. J., & Ferro, J. (1984). Contribución al estudio de los ecosistemas de arenas blancas. IV Reunión Científica de Profesores del Instituto Superior Pedagógico de Pinar del Río, Cuba. [ Links ]
Oehl, F., Sieverding, E., Ineichen, K., Mäder, P., Wiemken, A., & Boller, T. (2009). Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agriculture Ecosystems and Environment, 134, 257-268. [ Links ]
Oliveira, A. N. (2001). Fungos micorrízicos arbusculares e teores de nutrientes em plantas de cupuaçu e guaraná de um Sistema Agroflorestal na região de Manaus, AM (Master dissertation). Universidade Federal do Amazonas, Brasil. [ Links ]
Porter, W. M., Robson, A. D., & Abbott, L. K. (1987). Field survey of the distribution of VAM fungi in relation to soil pH. Journal of Applied Ecology, 24, 659-662. [ Links ]
Read, D. J., Koucheki, H. K., & Hodgson, J. (1976). Vesicular-arbuscular mycorrhiza in natural vegetation systems. I-The occurrence of infection. New Phytologist, 77, 641-653. [ Links ]
Ricardo, N., Herrera, P. P., Cejas, F., Bastart, J. A., & Regalado, T. (2009). Tipos y características de las formaciones vegetales de Cuba. Acta Botánica Cubana, 203, 1-42. [ Links ]
Rodríguez-Rodríguez, R. M. (2013). Potencialidades de uso de las micorrizas arbusculares en la conservación de plantas cubanas. Bissea, 7(3). [ Links ]
Rodríguez-Rodríguez, R. M., Herrera, P., & Furrazola, E. (2013). Arbuscular mycorrhizal colonization in Asteraceae from white sand savannas, in Pinar del Río, Cuba. Biota Neotropica, 13(3), 136-140. [ Links ]
Samek, V. (1973). Regiones fitogeográfitas de Cuba. Academia de Ciencias de Cuba. Serie Forestal, 15, 1-63. [ Links ]
Sanders, I. R. & Fitter, A. H. (1992). The ecology and functioning of vesicular-arbuscular mycorrhizas in co-existing grassland species I. Seasonal patterns of mycorrhizal occurrence and morphology. New Phytologist, 120, 517-524. [ Links ]
Schnoor, T. K., Lekberg, Y., Rosendahl, S., & Olsson, P. A. (2011). Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in natural grassland. Mycorrhiza, 21, 211-220. [ Links ]
Sigüenza, C, Espejel, I., & Allen, E. B. (1996). Seasonality of mycorrhizae in coastal sand dunes of Baja California. Mycorrhiza, 6, 151-157. [ Links ]
Trouvelot, A, Kough, J. L., & Gianinazzi-Pearson, V. (1986). Mesure du taux de mycorrhization VA d’un syste`me radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionelle. In V. Gianinazzi-Pearson, & S. Gianinazzi (Eds.), Physiological and Genetical Aspects of mycorrhiza (pp. 101-109). Paris: INRA. [ Links ]
Turrini, A., & Giovannetti, M. (2011). Arbuscular mycorrhizal fungi in national parks, nature reserves and protected areas worldwide: a strategic perspective for their in situ conservation. Mycorrhiza, 22, 81-97. [ Links ]
Urquiola, A. J., González-Olia, L., Novo, R., & Acosta, Z. (2010). Libro rojo de la flora vascular de la provincia de Pinar del Río. Cuba, Jardín Botánico de Pinar del Río: Publicaciones Universidad de Alicante. [ Links ]
Vilamajó, D. (1989). Mapa de Bioclima. La Habana, Cuba: Nuevo Atlas Nacional de Cuba. [ Links ]
Villate, M., Herrera, P. P., Urquiola, A. J., & Ricardo, N. E. (2010). Flora sinántropa en las comunidades terrestres de la Reserva Florística Manejada San Ubaldo-Sabanalamar, Pinar del Río, Cuba. Acta Botánica Cubana, 207, 35-44. [ Links ]
Wilson, G. T. W., & Hartnett, D. C. (1997). Effects of mycorrhizae on plant growth and dynamics in experimental tallgrass prairie microcosms. American Journal of Botany, 84, 478-482. [ Links ]
Yang, C., Hamel, C., Schellenberg, M. P., Perez, J. C., & Berbara, R. L. L. (2010). Diversity and functionality of arbuscular mycorrhizal fungi in three plant communities in semiarid grasslands National Park, Canada. Microbial Ecology, 59, 724-733. [ Links ]
1. Instituto de Ecología y Sistemática, Carretera Varona 11835 e/Oriente y Lindero, La Habana 19, CP 11900, Calabazar, Boyeros, La Habana, Cuba; eduardof@ecologia.cu
2. CONICET-INBIOTEC, FIBA Mar Del Plata/EEA INTA, Balcarce, Argentina; covacevich.fernanda@inta.gob.ar
3. Estación de Monitoreo y Análisis Ambiental ECOVIDA. Km 4 1/2 Carretera a La Fe, Sandino, Pinar del Río, Cuba; kizquierdomedero@gmail.com
4. Instituto de Investigaciones de Sanidad Vegetal. Calle 110 no. 514 e/ 5.a B y 5.a F, Playa, C.P. 11600, La Habana, Cuba; rigelmex13@hotmail.com
5. Universidade Federal Rural do Rio de Janeiro (UFRRJ), Instituto de Agronomia, Departamento do Solos, Laboratório de Biologia do Solos. Rio de Janeiro, Brasil; berbara@ufrrj.br
Received 03-VI-2014. Corrected 12-XI-2014. Accepted 09-XII-2014.














