Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.62 n.3 San José Jul./Sep. 2014
Species richness and relative species abundance of Nymphalidae (Lepidoptera) in three forests with different perturbations in the North-Central Caribbean of Costa Rica
Riqueza y abundancia relativa d especies de Nymphalidae (Lepidoptera) en tres bosques con diversos grados de alteración en el centro norte de Caribe Costa Rica
Riqueza y abundancia relativa d especies de Nymphalidae (Lepidoptera) en tres bosques con diversos grados de alteración en el centro norte de Caribe Costa Rica
Abstract
Measurements of species richness and species abundance can have important implications for regulations and conservation. This study investigated species richness and abundance of butterflies in the family Nymphalidae at undisturbed, and disturbed habitats in Tirimbina Biological Reserve and Nogal Private Reserve, Sarapiquí, Costa Rica. Traps baited with rotten banana were placed in the canopy and the understory of three habitats: within mature forest, at a river/forest border, and at a banana plantation/forest border. In total, 71 species and 487 individuals were caught and identified during May and June 2011 and May 2013. Species richness and species abundance were found to increase significantly at perturbed habitats (p<0.0001, p<0.0001, respectively). The edge effect, in which species richness and abundance increase due to greater complementary resources from different habitats, could be one possible explanation for increased species richness and abundance. Rev. Biol. Trop. 62 (3): 919-928. Epub 2014 September 01.
Key words: butterflies, disturbance effects, edge effect, nymphalidae, species abundance, species diversity, species richness, Chiquita Nature and Community Project, Nogal Private Reserve.
Resumen
La medición de la riqueza y abundancia de especies puede indicar la salud de un ecosistema y tener implicaciones importantes para la conservación y su regulación. Este estudio investigó la riqueza y abundancia de mariposas de la familia Nymphalidae en hábitats alterados y no alterados, primordialmente en la Reserva Biológica Tirimbina y en la Reserva Privada Nogal, Sarapiquí, Costa Rica. Se colocaron trampas cebadas con banano podrido en el dosel y sotobosque de tres hábitats: en un bosque primario, en el lindero entre un río y un bosque y en el lindero entre una plantación de bananos y un bosque. Se atrapó e identificó un total de 71 especies y 487 individuos durante mayo y junio 2011 y en mayo 2013. Se encontró que la riqueza y la abundancia de especies aumentaron significativamente en los hábitats de los linderos (p<0.0001, p<0.0001 respectivamente). El efecto de borde, según el cual la riqueza y la abundancia de especies aumentan a causa de la existencia de más recursos complementarios provenientes de dos hábitats distintos, podría ser una posible explicación del aumento en la riqueza y la abundancia de especies en hábitats alterados.
Palabras clave: abundancia de especies, diversidad de especies, efecto de alteración, mariposas, Nymphalidae, riqueza de especies, Proyecto Chiquita Naturaleza y Comunidad, Reserva Privada Nogal.
Biological diversity has become increasingly important in the realm of conservation biology. Changes in species richness and species relative abundance due to disruptions in continuous habitats have been used in multiple studies to suggest or evaluate management decisions on which conservation efforts are needed to maintain the survival of a species or a community of species (Horner-Devine, Daily, Ehrlich, & Boggs, 2003; Uehara-Prado, Brown, & Freitas, 2007; Sjödin, Bengtsson, & Ekbom, 2008; Hjältén et al., 2012).
Insects, and in particular butterflies, have contributed greatly to understanding tropical biodiversity (DeVries, Alexander, Chacon, & Fordyce, 2012). Although plentiful, insects are still incredibly prone to great population increases and decreases, and have been used frequently as important indicators of disturbance to ecosystems (Erhardt, 1985; Fleishman, Thomson, Nally, Murphy, & Fay, 2005; Bobo, Waltert, Fermon, Njokagbor, & Mühlengber, 2006; Pöyry et al., 2006; Leidner, Haddad, & Lovejoy, 2010). Habitat loss is thought to be the greatest cause for insect extinction, and their populations can be easily influenced by anthropogenic forest fragmentation (Hogue, 1993; Leidner et al., 2010). Barlow, Overal, Araujo, Gardner, & Peres (2007) found that butterfly species richness in Brazil tended to be greater in primary forest than on Eucalyptus plantations, but that relative species abundance of the species existing there tended to be greater on the Eucalyptus plantations. Uehara-Pradoet al. (2007) compared primary forest and fragmented forest in the Brazilian Atlantic, finding that butterfly species richness increased at fragmented sites while relative abundance did not change, suggesting that forest fragmentation and disturbance does not negatively affect species diversity. Horner-Devine et al. (2003) found that coffee farms within 1-2.5km of a forest reserve had greater butterfly species richness and relative species abundance than both, farms 6-9km away and within the forest reserve itself, indicating that nearby forest may have a positive impact on butterfly species richness and relative species abundance. It also suggests that forest edges may experience increases in species richness and abundance.
This study focuses on the butterfly family Nymphalidae, which contains more species than any other butterfly family in the world, except the family Lycaenidae. Nymphalidae species can be found on every continent except Antarctica, although they are most diverse in Neotropical regions (DeVries, 1987). More specifically, this study looked at fruit-feeding nymphalids, a feeding guild in Nymphalidae, which feed on rotten fruit juices (DeVries et al., 2012). The objectives of this study were to compare species richness and relative species abundance in the butterfly family Nymphalidae in both the canopy and the understory between (a) mature forest habitat and two disturbance habitats: (b) river/forest (a natural disturbance) and (c) banana plantation/forest (an anthropogenic disturbance).
Materials and Methods
Study site: This study took place primarily in Tirimbina Biological Reserve (referred to as TBR in this study), located in the second district of La Virgen, Sarapiquí, Heredia Province in the North central-Caribbean area of Costa Rica. La Virgen has a population of 8 715 people, and is a rural area (Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital, 2012b). The TBR (345ha) is located in the Sarapiquí River Basin of approximately 1 923km2 in size. It is bordered by other private properties that altogether create 600ha of continuous forest cover (Tirimbina Biological Reserve, 2010a). The TBR (10°24’ N and 84°7’ W) is at an elevation of 180-220m above sea level and is made up of 345ha of lowland rainforest. Mean annual temperature is 25.3°C with a mean high of 30.2°C and a mean low of 20.2°C. Mean annual precipitation is 3777mm. Landscape inclines generally fall between 10-25% with rolling hills, although closer to creeks and the river, slopes can reach 60%. Soils are of volcanic origin. The Sarapiquí River forms the Western border of the reserve. Tirimbina falls within two Holdridge life zones, Humid Tropical Pre-Montane Forest and transition to Basal and Humid Tropical Forest. Primary forest makes up 85% of TBR while the rest is secondary forest (Tirimbina Biological Reserve, 2010b).
The data of the banana plantation/forest border was part of a long term study executed by Chiquita Nature and Community Project at Nogal Private Reserve and forest fragments around it. This fragment is located close to Puerto Viejo, Sarapiquí at 40-60m elevation. Mean annual precipitation falls between 3 712-4 000mm. The mean annual temperature is 25.8°C, and the vegetation corresponds to Holdridge Life Zone of Humid Tropical Forest (Barquero Villalobos, 2009). Although close to Puerto Viejo, the surrounding area of this plantation is rural with fragmented forest. In general, banana and pineapple farms are especially common in Sarapiquí (Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital, 2012a).
Data collection: In TBR, data was taken from a river/forest border along the Río Sarapiquí and from within the forest itself. In TBR, data was taken 5d/wk for 3 weeks and 4d the 4th week between 2 to 31 May. Data used from the banana plantation/forest border were taken 3-7 May and 31 May-5 June, 2011 by staff of Chiquita Nature and Community Projects as part of their long-term butterfly survey.
Traps were made out of mosquito netting held in a cylindrical shape with an open bottom and closed top approximately 30cm in diameter and 1m in height as described in DeVries (1987). Between 50 and 100mL of rotten banana were placed in a small plastic cup on a platform hanging about 5-8cm from the open bottom of the trap. Banana was mashed and left to sit about 1d before baiting. Traps were re-baited with banana only as necessary throughout the week. Traps were checked once/d (trap-day). All butterflies captured during a trap-day were counted by number of individuals of each species and identified using DeVries (1987). Collected specimens were given to the Instituto Nacional de Biodiversidad (INBio), Santo Domingo de Heredia, Costa Rica. Data on precipitation levels each day were obtained from TBR. Sampling from the banana plantation study was conducted in a similar manner.
Twenty traps were set up at the river/forest border throughout the entire study. For the first week of the study, 30 traps within the forest were used from another ongoing study. For the next 3wks, 20 different traps were set up and used to collect forest data. In each habitat, traps were located at 10 or 15 paired points with one trap in the canopy and one in the understory. Traps in both perturbed habitats were set up inside the forest near the border, though not necessarily right on the tree line.
Differences in: a) mean number of species captured per trap-day and b) mean number of individuals per species per trap-day were estimated between canopy and understory both within and between habitats with two-way parametric ANOVA. A posteriori comparisons between means were tested with Scheffe intervals. Parametric test assumptions were carried out before executing ANOVAS and procedures followed Sokal and Rohlf (1995). Statistical analyses were carried out with Statgraphics XV.I software (Statpoint Technologies, Inc., 2011).
Brillouin diversity indexes and Brillouin evenness indexes were calculated using Microsoft Excel 2007 Diversity Addin. Uneven sample sizes were accounted for by calculating the diversity index for each trap-day and checking for significance through an ANOVA test with Statgraphics XVI (Statpoint Technologies, Inc., 2011).
A species accumulation curve was created to estimate the proportion of butterflies identified to the actual number of species in the population. Relationship between daily precipitation data versus daily captures of number of species and number of individuals was estimated with simple regression carried out with Statgraphics XV.I (Statpoint Technologies, Inc. 2011). A regression was not done for the banana plantation/forest habitat due to differences in data collection on precipitation A “square-root-Y” model (best fit) was used in the mature forest habitat and a “double-squared” model (best fit) was used in the river/forest border habitat for number of species. A “square root-Y” model (best fit) was used in the forest habitat and a “squared-Y” model (best fit) in the river/forest border habitat for number of individuals. Simper Analysis was used to evaluate changes in species composition with the PAST program (Hammer, Harper, & Ryan, 2001).
Results
In total, 71 species and 487 individuals were caught and identified (Table 1). In general the three most abundant species were Cithaerias pireta pireta, Hamadryas laodamia saurites, and Caligo brasiliensis sulanus. When comparing the five most abundant species in each habitat, only two species were found in more than one habitat: Catonephele orites and Colobura dirce dirce (Table 2). At the genus level, only three were found in two habitats: Hamadryas (forest and river), Catonephele (forest and banana plantation), and Colobura (forest and banana plantation).
A total of 36 species were only found in one habitat: 8 in mature forest habitat, 21 in the river/forest border habitat and 7 in the banana plantation/forest border habitat (Table 3). For 20 of those species, only one individual was found.
Comparison of number of species: Mean number of species captured per trap-day was greatest in the banana plantation/forest border habitat (
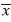 =0.74) and least in mature forest habitat (
=0.74) and least in mature forest habitat (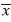 =0.254) with the river/forest border habitat as an intermediary (
=0.254) with the river/forest border habitat as an intermediary (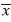 =0.511) (F=29.1; df=2, 997; p<0.0001). Overall, mean species number captured per trap-day was greater in the canopy (
=0.511) (F=29.1; df=2, 997; p<0.0001). Overall, mean species number captured per trap-day was greater in the canopy (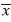 =0.626) compared to the understory (
=0.626) compared to the understory (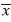 =0.379) when observations of all habitats were combined (F=24.8; df=1, 994; p<0.0001). However, this trend was not the same for each individual habitat (F=16.07; df=2, 994; p<0.0001, Fig. 1).The river/forest border habitat had the greatest difference between mean number of species between the canopy and the understory and no difference existed for the banana plantation/forest border habitat. Mean species number was greater in canopy than understory in mature forest habitat, but not significantly so. The lack of parallel trends in figure 1 indicates that habitat plays a strong impact on changing differences between mean number of species in the canopyand understory.
=0.379) when observations of all habitats were combined (F=24.8; df=1, 994; p<0.0001). However, this trend was not the same for each individual habitat (F=16.07; df=2, 994; p<0.0001, Fig. 1).The river/forest border habitat had the greatest difference between mean number of species between the canopy and the understory and no difference existed for the banana plantation/forest border habitat. Mean species number was greater in canopy than understory in mature forest habitat, but not significantly so. The lack of parallel trends in figure 1 indicates that habitat plays a strong impact on changing differences between mean number of species in the canopyand understory. Species accumulation curve: The number of species accumulated in mature forest appeared to begin to plateau towards the end of the last sampling period, indicating that this study may have identified the majority of Nymphalidae species in that habitat. However, in both the river/forest habitat and the banana plantation/forest habitat, the number of identified species appeared to continue sloping upwards, indicating that there may still have been several unidentified species, leaving the final data incomplete.
Comparison of number of individuals: Mean number of individuals was greatest in the banana plantation/forest border habitat (
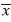 =0.875) and least in the mature forest habitat (
=0.875) and least in the mature forest habitat (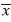 =0.263) with the river/forest border habitat as an intermediary (
=0.263) with the river/forest border habitat as an intermediary (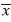 =0.534) (F=32.3; df=2, 997; p<0.0001, Fig. 2). Overall, mean number of individuals captured per trap-day was greater in the canopy (
=0.534) (F=32.3; df=2, 997; p<0.0001, Fig. 2). Overall, mean number of individuals captured per trap-day was greater in the canopy (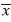 =0.650) than the understory (
=0.650) than the understory (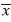 =0.467) when observations of all habitats were combined (F=9.93; df=1, 994; p=0.0016), although the banana plantation/forest border habitat strayed from this pattern when looking at each habitat (F=19.2; df=2, 994; p<0.0001, Fig. 3). The river/forest border was the only habitat with a significant difference between the mean number of individuals in canopy and understory. The lack of parallel trends in figure 3 indicates that habitat plays a strong impact on changing differences between mean number of individuals in the canopy and the understory.
=0.467) when observations of all habitats were combined (F=9.93; df=1, 994; p=0.0016), although the banana plantation/forest border habitat strayed from this pattern when looking at each habitat (F=19.2; df=2, 994; p<0.0001, Fig. 3). The river/forest border was the only habitat with a significant difference between the mean number of individuals in canopy and understory. The lack of parallel trends in figure 3 indicates that habitat plays a strong impact on changing differences between mean number of individuals in the canopy and the understory. Brillouin and Brillouin Evenness Diversity indices: The Brillouin Diversity Index indicated significantly different mean indices between habitats. The banana plantation/forest habitat had the greatest diversity (
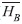 =0.082), the mature forest habitat had the smallest diversity (
=0.082), the mature forest habitat had the smallest diversity (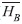 =0.013), and the river/forest border was intermediary (
=0.013), and the river/forest border was intermediary (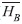 =0.055). The Brillouin Evenness Index had similar results; the banana plantation/forest habitat had the greatest index (
=0.055). The Brillouin Evenness Index had similar results; the banana plantation/forest habitat had the greatest index (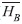 =0.18), the river/forest border as intermediary (
=0.18), the river/forest border as intermediary (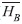 =0.12), and the mature forest habitat with the smallest index (
=0.12), and the mature forest habitat with the smallest index (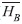 =0.35).
=0.35). Simper Analysis revealed that Cithaerias pireta pireta contributed the most to dissimilarity between groups (% Contribution=7.769%) with a relatively great abundance in the banana plantation/forest border habitat (mean abundance=0.215) and relatively smaller abundances in the river/forest border habitat (mean abundance=0) and in the mature forest habitat (mean abundance=0.00941). Hamadryas laodamia saurites (Contribution %=3.094%), Caligo brasiliensis sulanus (Contribution %=2.87%), and Nessaea aglaura aglaura (Contribution %=2.858%) also had greater contributions to dissimilarity.
Comparison with captures versus precipitation: In the mature forest habitat, the number of species captured/day decreased significantly with increased daily precipitation (F=8.63; df=1, 17; p=0.0092; R2=33.68%). Number of species captured/day in the river/forest border habitat also decreased with increased precipitation, but not significantly (F=1.17; df=1, 17; p=0.2955; R2=6.414%).
Discussion
Species richness and species abundance increased in the habitats of disturbance, with the greatest numbers in the banana plantation/forest border habitat. Brillouin Diversity Index and Brillouin Evenness Index indicated greater butterfly diversity in habitats of disturbance as well. Species richness and abundance tended to be greater in the canopy than in the understory. Previous studies have indicated that borders between two different kinds of habitat can attract or repel different butterfly species, creating something called “edge effects”. According to Ries, Sisk, and Karban (2008), butterfly density tends to increase at the border between two habitats when there is a strong difference between habitat quality and distribution of necessary or complementary resources. DeVries (1987) attributed this increased species richness and relative species abundance at forest edges to similarities between canopies and forest edges, such as more light and a more dynamic and diverse physical environment. Tree gaps within continuous rain forest may also experience canopy butterfly species near the ground due to changes in vegetative structure (Pardonnet, Beck, Milberg, & Bergman, 2013), reaffirming that areas of disturbance may contain similar characteristics to the canopy, and thus attract more of those species.Horner-Devine et al. (2003) hypothesized that increases near borders could be due to increased microclimates that include necessary host plants and food, as well as increased space for movement.
Of the 71 species identified, 36 (50.7%) of those species were found in only one habitat. Of the five most abundant species found in each habitat, only two in total were found present in more than one habitat. The top contributors to dissimilarity according to the Simper Analysis were also all species within the five most abundant in each habitat. This suggests that each habitat provides key services to specific species and has strong effects on species composition, even though each of these habitats includes primary forest and all are found in the same region. Pardonnet et al. (2013) found that tree gaps in Peruvian rain forests caused changes in butterfly assemblages when compared to adjacent nearby understory, indicating that species composition may change within the same continuous forest should vegetative structure vary, as it does at habitat borders.
Increased precipitation negatively affected the number of species and individuals significantly in mature forest habitat. Although not significantly affected, number of species and individuals did tend to decrease with increased rainfall in the river/forest border habitat as well. This could be because rainfall is not conducive to flight (Raupp, 2006). Precipitation levels could account for high levels of variance in samples. It also suggests that species abundance is affected by more than just habitat.
Limitations to my study may include error generated from temporal and location differences in data collection. Data from the banana plantation/border habitat came from a study site 20km from TBR and was collected 2yrs before the data collected for the other two habitats. In the first week of data collection in the mature forest habitat, traps were set up in different locations than in weeks 2-4 due to a lack of available traps, creating more chance for error. This study was also conducted in May, during which the dry season usually transitions into the rainy season, which may have affected changes in butterfly species and abundance.
Future research may want to account for changes in precipitation levels and changes in species composition over increased time periods. May tends to be the transition between the rainy season and the dry season in Sarapiquí, and there might be differences between rainy season data and dry season that are worth comparing, especially because commonality of some species seemed to change from the beginning of the month to the end. Therefore, results may vary depending on the time of year. As indicated by the increasing curves in figure 4, a longer sampling period may also be needed to obtain a complete picture of species composition in each habitat.
Acknowledgments
Special thanks to Christian Miranda for teaching me all about butterfly identification and accompanying me so many times in the field. We thank the Chiquita Nature and Community Project for allowing us to use data from their own research project. We also thank Isidro Chacón for helping us with species identification and everyone at the ACM office, most especially Michael McCoy and Chris Vaughan for guidance on our project and review of this paper, as well as Mario Morera for helping the first author in Spanish translations.
References
Barlow, J., Overal, W. L., Araujo, I. S., Gardner, T. A., & Peres, C. A. (2007). The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. Journal of Applied Ecology, 44, 1001-1012. [ Links ]
Barquero Villalobos, K. (2009). Corredor Biológico Local Nogal-La Selva. Chiquita Nature and Community Project. Retrieved from http://www.nogalnatureandcommunity.com/ndex.php/en/conservation/biological-corridors [ Links ]
Bobo, K. S., Waltert, M., Fermon, H., Njokagbor, J., & Mühlengber, M. (2006). From forest to farmland: Butterfly diversity and habitat associations along a gradient of forest conversion in southwestern Cameroon. Journal of Insect Conservation, 10, 29-42. [ Links ]
DeVries, P. J., Alexander, L. G., Chacon, I. A., & Fordyce, J. A. (2012). Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. Journal of Animal Ecology, 81(2), 472-482. [ Links ]
DeVries, P. J. (1987). The butterflies of Costa Rica and their natural history: Papilionidae, Pieridae, Nymphalidae. Princeton, NJ, USA: Princeton University. [ Links ]
Erhardt, A. (1985). Diurnal Lepidoptera: Sensitive indicators of cultivated and abandoned grasslands. Journal of Applied Ecology, 22(3), 849-861. [ Links ]
Fleishman, E., Thomson, J. R., Nally, R. M., Murphy, D. D., & Fay, J. P. (2005). Using indicator species to predict species richness of multiple taxonomic groups. Conservation Biology, 19(4), 1125-1137. [ Links ]
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1), 9. [ Links ]
Hjältén, J., Stenbacka, F., Pettersson, R. B., Gibb, H., Johansson, T., Danell, K., & Hilszczański, J. (2012). Micro and macro-habitat associations in saproxylic beetles: Implications for biodiversity. PLoS ONE, 7(7), 1-11. [ Links ]
Horner-Devine, M. C., Daily, G. C., Ehrlich, P. R., & Boggs, C. L. (2003). Countryside biogeography of tropical butterflies. Conservation Biology, 17(1), 168-177. [ Links ]
Hogue, C. L. (1993). Latin American Insects and Entomology. Berkeley, California, USA: University of California. [ Links ]
Leidner, A. K., Haddad, N. M., & Lovejoy, T. E. (2010). Does tropical forest fragmentation increase long-term variability of butterfly communities? PLoS ONE, 5(3), 1-8. [ Links ]
Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital. (2012a). Datos socioeconómicos y ambientales. Municipalidad de Sarapiquí. Retrieved from http://www.sarapiqui.go.cr/index.php?option=com_content&view=article&id=137:datos-socioeconomicos-y-ambientales&catid=12&Itemid=254 [ Links ]
Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital. (2012b). Información poblacional. Municipalidad de Sarapiquí. Retrieved from http://www.sarapiqui.go.cr/index.php?option=com_content&view=article&id=136:informacionoblacional&catid=12&Itemid=254 [ Links ]
Pardonnet, S., Beck, H., Milberg, P., & Bergman, K. (2013). Effect of Tree-Fall Gaps on Fruit-Feeding Nymphalid Butterfly Assemblages in a Peruvian Rain Forest. Biotropica, 45(5), 612-619. [ Links ]
Pöyry, J., Luoto, M., Paukkunen, J., Pykälä, J., Raatikainen, K., & Kuussaari, M. (2006). Different responses of plants and herbivore insects to a gradient of vegetation height: An indicator of the vertebrate grazing intensity and successional age. OIKOS, 115, 401-412. [ Links ]
Raupp, M. (2006). What do butterflies do when it rains? Scientific American. Retrieved from http://www.scientificamerican.com/article.cfm?id=what-do-butterflies-do-wh [ Links ]
Ries, L., Sisk, T. D., & Karban, R. (2008). Butterfly edge effects are predicted by a simple model in a complex landscape. Oecologia, 156(1), 75-86. [ Links ]
Sjödin, N. E., Bengtsson, J., & Ekbom, B. (2008). The influence of grazing intensity and landscape composition on the diversity and abundance of flower-visiting insects. Journal of Applied Ecology, 45(3), 763-772. [ Links ]
Sokal, R. R. & Rohlf, F. J. (1995). Biometry: The principles and practice of statistics in biological research. New York, NY, USA: Wiley. [ Links ]
Statpoint Technologies, Inc. (2011). Statgraphics XV.I Statgraphics Centurion. Retrieved from http://www.statgraphics.com/downloads.htm [ Links ]
Tirimbina Biological Reserve. (2010a). Conservation Efforts. Tirimbina. Retrieved from Tirimbina website http://www.tirimbina.org/conservation.html [ Links ]
Tirimbina Biological Reserve. (2010b). Physical Description. Tirimbina. Retrieved from Tirimbina website http://www.tirimbina.org/what-is-tirimbina/physical-description.html [ Links ]
Uehara-Prado, M., Brown, K. S. Jr., & Freitas, A. V. L. (2007). Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic forest: Comparison between a fragmented and a continuous landscape. Global Ecology and Biogeography, 16(1), 43-54. [ Links ]
Barquero Villalobos, K. (2009). Corredor Biológico Local Nogal-La Selva. Chiquita Nature and Community Project. Retrieved from http://www.nogalnatureandcommunity.com/ndex.php/en/conservation/biological-corridors [ Links ]
Bobo, K. S., Waltert, M., Fermon, H., Njokagbor, J., & Mühlengber, M. (2006). From forest to farmland: Butterfly diversity and habitat associations along a gradient of forest conversion in southwestern Cameroon. Journal of Insect Conservation, 10, 29-42. [ Links ]
DeVries, P. J., Alexander, L. G., Chacon, I. A., & Fordyce, J. A. (2012). Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. Journal of Animal Ecology, 81(2), 472-482. [ Links ]
DeVries, P. J. (1987). The butterflies of Costa Rica and their natural history: Papilionidae, Pieridae, Nymphalidae. Princeton, NJ, USA: Princeton University. [ Links ]
Erhardt, A. (1985). Diurnal Lepidoptera: Sensitive indicators of cultivated and abandoned grasslands. Journal of Applied Ecology, 22(3), 849-861. [ Links ]
Fleishman, E., Thomson, J. R., Nally, R. M., Murphy, D. D., & Fay, J. P. (2005). Using indicator species to predict species richness of multiple taxonomic groups. Conservation Biology, 19(4), 1125-1137. [ Links ]
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1), 9. [ Links ]
Hjältén, J., Stenbacka, F., Pettersson, R. B., Gibb, H., Johansson, T., Danell, K., & Hilszczański, J. (2012). Micro and macro-habitat associations in saproxylic beetles: Implications for biodiversity. PLoS ONE, 7(7), 1-11. [ Links ]
Horner-Devine, M. C., Daily, G. C., Ehrlich, P. R., & Boggs, C. L. (2003). Countryside biogeography of tropical butterflies. Conservation Biology, 17(1), 168-177. [ Links ]
Hogue, C. L. (1993). Latin American Insects and Entomology. Berkeley, California, USA: University of California. [ Links ]
Leidner, A. K., Haddad, N. M., & Lovejoy, T. E. (2010). Does tropical forest fragmentation increase long-term variability of butterfly communities? PLoS ONE, 5(3), 1-8. [ Links ]
Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital. (2012a). Datos socioeconómicos y ambientales. Municipalidad de Sarapiquí. Retrieved from http://www.sarapiqui.go.cr/index.php?option=com_content&view=article&id=137:datos-socioeconomicos-y-ambientales&catid=12&Itemid=254 [ Links ]
Municipalidad de Sarapiquí-Secretaria Técnica de Gobierno Digital. (2012b). Información poblacional. Municipalidad de Sarapiquí. Retrieved from http://www.sarapiqui.go.cr/index.php?option=com_content&view=article&id=136:informacionoblacional&catid=12&Itemid=254 [ Links ]
Pardonnet, S., Beck, H., Milberg, P., & Bergman, K. (2013). Effect of Tree-Fall Gaps on Fruit-Feeding Nymphalid Butterfly Assemblages in a Peruvian Rain Forest. Biotropica, 45(5), 612-619. [ Links ]
Pöyry, J., Luoto, M., Paukkunen, J., Pykälä, J., Raatikainen, K., & Kuussaari, M. (2006). Different responses of plants and herbivore insects to a gradient of vegetation height: An indicator of the vertebrate grazing intensity and successional age. OIKOS, 115, 401-412. [ Links ]
Raupp, M. (2006). What do butterflies do when it rains? Scientific American. Retrieved from http://www.scientificamerican.com/article.cfm?id=what-do-butterflies-do-wh [ Links ]
Ries, L., Sisk, T. D., & Karban, R. (2008). Butterfly edge effects are predicted by a simple model in a complex landscape. Oecologia, 156(1), 75-86. [ Links ]
Sjödin, N. E., Bengtsson, J., & Ekbom, B. (2008). The influence of grazing intensity and landscape composition on the diversity and abundance of flower-visiting insects. Journal of Applied Ecology, 45(3), 763-772. [ Links ]
Sokal, R. R. & Rohlf, F. J. (1995). Biometry: The principles and practice of statistics in biological research. New York, NY, USA: Wiley. [ Links ]
Statpoint Technologies, Inc. (2011). Statgraphics XV.I Statgraphics Centurion. Retrieved from http://www.statgraphics.com/downloads.htm [ Links ]
Tirimbina Biological Reserve. (2010a). Conservation Efforts. Tirimbina. Retrieved from Tirimbina website http://www.tirimbina.org/conservation.html [ Links ]
Tirimbina Biological Reserve. (2010b). Physical Description. Tirimbina. Retrieved from Tirimbina website http://www.tirimbina.org/what-is-tirimbina/physical-description.html [ Links ]
Uehara-Prado, M., Brown, K. S. Jr., & Freitas, A. V. L. (2007). Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic forest: Comparison between a fragmented and a continuous landscape. Global Ecology and Biogeography, 16(1), 43-54. [ Links ]
1. Knox College, Galesburg, IL 61401, USA; cstephen@knox.edu
2. Asociación Theria para la Investigación y Conservación, San José, Costa Rica; ragde1578@yahoo.com.mx
Received 20-iii-2014. Corrected 30-iv-2014. Accepted 26-v-2014.














