Revista de Biología Tropical
versão On-line ISSN 0034-7744versão impressa ISSN 0034-7744
Rev. biol. trop vol.62 no.2 San José Abr./Jun. 2014
Trophic analysis of three species of Marilia (Trichoptera: Odontoceridae) from the neotropics
Análisis trófico de tres especies de Marilia (Trichoptera: Odontoceridae) del neotrópico
Análisis trófico de tres especies de Marilia (Trichoptera: Odontoceridae) del neotrópico
*Dirección para correspondencia:
Abstract
The trophic ecology of the aquatic insect fauna has been widely studied for the Northern temperate zone. However, the taxa originally classified within a given particular trophic group in temperate ecosystems, do not necessarily exhibit the same dietary profile beyond its geographic limits. Since, the trophic ecology of caddisfly larvae is largely incomplete in the Neotropical Region, the present work aims to describe feeding habits inferred from quantitative analysis of data taxonomically resolved at the species level. For this, the feeding habits of three Trichoptera species Marilia cinerea, M. elongata and M. flexuosa were recorded in the Yungas forests of Argentina and Bolivia. A total of 15 larvae of each species were sampled from 13 different streams were selected for gut content analysis. The ingested material was extracted from the foregut and midgut by using ventral dissection of thorax. For each species, mandibles were dissected, mounted in glycerin and illustrated in order to highlight morphological differences between these mouth pieces purportedly associated to the dietary behavior of individuals, and their habitats. The niche overlap was estimated through Schoener’s method. The diet analysis revealed that M. cinerea, M. elongata and M. flexuosa feed on the same food items, but through different patterns of preferences. Larvae of M. cinerea were collected on both emerging surfaces of rocks on which a thin layer of running water flows and streams sliding areas with stony bottoms attached to the rock surfaces. They displayed a gut content consisting predominantly of invertebrate vestiges and have strong mouthparts provided of large molar areas; this allowed us to allocate the species within the functional group of predators. M. elongata feeds mainly on fine particulate material, its mouthparts are scoop-shaped and occurs in areas of low flow; this set of features is linked to a collector-gatherer strategy. Finally, larvae of M. flexuosa have been primarily assigned to the functional group of shredders and secondarily to the collector-gatherer class. They inhabit sandy bottoms of mountain streams, have strong scoop-shaped mouthparts and show a diet dominated by leaf litter and fine particulate material. We concluded that the functional group assignment to the genus level for Marilia is not recommended, and further studies at species level are necessary. Rev. Biol. Trop.62 (2): 543-550. Epub 2014 June 01.
Key words: functional feeding group, Marilia cinerea, Marilia elongata, Marilia flexuosa, Schoener’s index.
Resumen
La ecología trófica de insectos acuáticos ha sido desarrollada en zonas templadas. Sin embargo, la asignación de un taxón a un grupo trófico no representa necesariamente el mismo en otras regiones. En la Región Neotropical, el conocimiento sobre la ecología trófica de larvas de tricópteros es incompleta y la literatura sobre este tema rara vez se ocupa del análisis de los hábitos alimentarios de larvas con datos cuantitativos a nivel de especie. Este trabajo aporta al conocimiento de las características tróficas de la trichopterofauna del Neotrópico. Se describen los hábitos alimentarios de tres especies de Trichoptera registrados en arroyos de Yungas de Argentina y Bolivia: Marilia cinerea, M. elongata y M. flexuosa. Se seleccionaron 15 larvas de cada especie en 13 arroyos de Yungas surandinas para analizar las piezas bucales y el contenido estomacal. El solapamiento de nicho trófico se estimó mediante el índice de Schoener. El análisis de la dieta reveló que las especies difieren en la preferencia de los ítems registrados, hábitat y forma de sus mandíbulas. Las larvas de M. cinerea habitan en superficies de rocas emergentes. Poseen mandíbulas fuertes con grandes zonas molares y consumen principalmente invertebrados. Esta evidencia permite asignarle el grupo funcional depredador. M. elongata consume material fino, sus mandíbulas tienen forma de cuchara y su ubicación en zonas de bajo flujo permite asignarle una estrategia colectora-recolectora. Las larvas de M. flexuosa habitan en fondos arenosos de arroyos de montaña, tienen fuertes piezas bucales en forma de cuchara y una dieta dominada por hojarasca y material fino. Pertenecen al grupo funcional triturador, secundariamente colector-recolector. Sugerimos que la asignación de grupo funcional a nivel de género no es recomendable para Marilia. Se recomiendan mayores estudios a nivel de especie.
Palabras clave: grupo funcional alimentario, Marilia cinerea, Marilia elongata, Marilia flexuosa, índice de Schoener.
The knowledge about the functional feeding groups of aquatic insects that inhabit lotic environments contributes to a right comprehension of the ecology of such ecosystems (Cummins, 1973). This knowledge is critical for understanding both (i) the energy pathways (nutrients cycling) and (ii) the resident entomofauna structure (community composition). In the organismic scale of analysis, the accurate definition of trophic traits is also important because these traits influences every vital aspect of individuals such as life cycles, habitat choice, agonistic behavior, food search, reproductive fitness, among others. The correct assignment of species to functional feeding groups depends upon evidence taken from three empirical levels of information: 1) the kind of ingested food item; 2) the behavioral strategy oriented to obtain resources; 3) the nature of the morphological structure for alimentary acquisition. Aquatic insects have been assigned to four different feeding categories, namely collectors (gatherers/filterers), shredders, scrapers and predators. The bulk of work on trophic ecology and functional classification of aquatic insects has been performed in the temperate zone (Merrit & Cummins, 1996). However, direct extensions of those results to other regions such us the Tropics of the New World could be misleading. Several authors have warned about the risks of the free importation of classificatory schema since taxa from temperate ecosystems do not necessarily match their dietary habits with those from the outside (Dobson, Magana, Mathooko & Ndegwa, 2002; Cheshire, Boyero & Pearson, 2005; Tomanova, Goitia & Helešic, 2006; Reynaga, 2009; Chará-Serna, Chará, Zúñiga, Pedraza & Giraldo, 2010).
In the Neotropical Region, the knowledge about ecology, behaviour or life history of caddisfly larvae is largely incomplete (Posada-García & Roldán Pérez, 2003). The available literature based on feeding habits is focused to the genus level (Cummins, Merrit & Andrade, 2005; Rincón & Martínez, 2006; Tomanova et al., 2006; Reynaga, 2009) and a few to the species level (Albariño & Valverde, 1998; Díaz-Villanueva & Trochine, 2005; Reynaga & Rueda-Martín, 2010). In this region, Marilia (Trichoptera: Odontoceridae) is the most specious genus in the family, with about 30 species. As all the Integripalpian larvae, they build transportable cases using silk. Portable cases are well suited to a foraging lifestyle in which the insects move throughout in search of food. Regarding this background, the aim is to study the feeding habits of three species belonging to this genus that occur in the mountain rain forests, or Yungas ecoregion of Argentina and Bolivia (Rueda-Martín, 2008): Marilia cinerea Navás 1931, M. elongata Martynov 1912 and M. flexuosa Ulmer 1905.
This study was carried out in order to analyze the functional feeding group of these species of Marilia present in Northwestern Argentina. We considered three different aspects: (1) where the food was obtained, focusing on the habitat where the larva was found, (2) what kind of material was ingested, animal, vegetal, and other items, and (3) morphological aspects of mouthparts.
The knowledge on the functional feeding groups of aquatic insects constitutes an important tool in biomonitoring programs. Moreover, it provides a basic knowledge for the identification of policies and proposal for conservation and maintenance use of natural resources of a given area.
Material and methods
Study material: Marilia cinerea is distributed in mountain and plane areas from central and Northwestern Argentina and Bolivia (from 400m to upper than 1 500m). The general coloration of the larva is light brown with some muscular scales (Fig. 1A); the head is rounded and bears a semicircular crest and has an average body length of 9.42±0.9mm. Marilia elongata is recorded from Peru and Northwestern Argentina. Some of the larvae have unusual cases in which the posterior portion is made of a case of a larva from another genus (Leptoceridae: Grumichella). The larvae are dark colored, with oval head bearing two triangular white spots and white area around the eyes (Fig. 1B) and have an average body length of 10.31±1.5mm. Marilia flexuosa is widely distributed from North America to Northwestern Argentina. The general color of the larval sclerites is dark with a dorsal carina and lateral bridges that cross the ocular area (Fig.1C). Larvae have an average body length of 6.40±0.7mm.
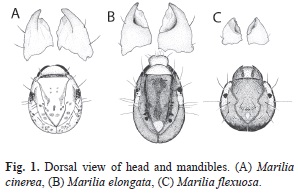
Diet analysis: Larvae of the three species were collected in 13 streams of Southern Andean Yungas. For each study site and sampling occasion, qualitative samples were taken from riffles and pools using a strainer (Rueda- Martín, 2008). The samples were preserved in 75% ethyl alcohol. In the laboratory, the individuals were identified to species level. A total of 15 larvae of each species were selected for gut content analysis from different sites. The diet was analyzed by removing the foregut and midgut content using ventral dissection of thorax (Peckarsky, 1996). The description and identification of ingested items were made under a microscope (200X). The gut content of each specimen was mounted with glycerin. The scale bar of the microscope eyepiece was used as a transect. A total of 15 transects were randomly selected, where each food category was recorded at each sampling point and its coverage percentage was estimated according to Reynaga & Rueda-Martín (2010). The food categories considered were: sediment particles, fine particulate organic matter (FPOM<1mm), coarse particulate organic matter (CPOM>1mm), microphyte and invertebrate remains. The mandibles of one individual of each species were dissected, mounted in glycerin and illustrated under microscope with a camera lucida to record possible differences in morphology and possible association with dietary items.
The niche overlap was calculated via Schoener’s (1970) method:
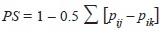
where pij and qik represent the fraction of food item i that is used by species j and k respectively, with regards to the total of food items. The index ranges from 0 which indicates no dietary overlap to a maximum overlap of 1 when all items are found in equal proportions (Jaksic, 2001) across the dietary profile. All statistical analyses and graphics were produced through the R platform (R Core Team, 2012).
Results
The diet analysis shows that M. cinerea, M. elongata and M. flexuosa consume the same food items, but in different proportions (Table 1). In general, invertebrate remains were the dominant item in M. cinerea; M. elongata feeds on amorphous matter and fine particulate organic matter; the diet of M. flexuosa is mainly characterized by particulate organic matter both coarse and fine (Fig. 2). In the latter two species it also should be also stressed the occurrence of sediment particles (>15%).
When analyzing their habitats and shapes of the mandibles differences were found between species. Larvae of M. cinerea were associated to the upper surface of rocks in mountain streams, in areas of running waters provided of periphyton or in gravelly bottoms. Their mandibles are stout; they have an apical blade ending in a blunt toothed edge and moderately large inner grinding areas (Fig. 1A). The M. elongata and M. flexuosa larvae were found in sandy areas of mountain streams with low flow velocity. Their mandibles are stout and the molar area is concave like a basin or scoop (Fig. 1B and 1C).
The complete linkage dendrogram (Fig. 3) obtained from the functional dissimilarity between taxa can be partitioned into three groups. The first group includes individuals of M. cinerea, assigned to the predator functional group. The second group comprises individuals of M. flexuosa, shredder primarily and collector-gatherers secondarily, and the last group composed by M. elongata, collector-gatherers specialized in amorphous matter and fine particulate organic material. This analysis suggests that there is no overlap in diet between the species studied. According to the dietary profile of individuals, species were grouped consistently regardless of the date or site where they were collected.
Discussion
Generally, studies on functional feeding groups of macroinvertebrates focus on the genus level. In this study, we analyzed the diet of the three species of Marilia registered for the Yungas region of Argentina and Bolivia, finding that both diet and habitat preferences are different. Larvae of M. cinerea were collected on emerging surface of rocks on which a thin layer of water runs or rivers sliding areas with stony bottoms, attached to the rock surfaces. The larvae displayed a diet of predominantly invertebrate remains; their mouthparts are strong with large molar areas, features which allow us to allocate this species within the functional group of predators. M. elongata feeds mainly on fine material, their mouthparts have scoop shape and their location in low flow area is associated with a collector-gatherer strategy. Larvae of M. flexuosa are associated to sandy bottoms in mountain streams. They have a diet composed of leaf litter and fine particulate material, their mouthparts are strong and scoop-shaped. The functional group assignment for this species corresponds to a shredder, mainly, and collector-gatherer, secondarily.
Wiggins (2004) suggests that the Odontocerid larvae in North America are primarily detritivores. Analysis of gut contents suggest that the larvae are omnivorous, feeding on detritus, algae, and animal materials, although the presence of insect parts in the gut contents could reflect predation. Oliveira & Froehlich (1997) and Henriques-Oliveira & Nessimian (2010) determined the functional group of Marilia of southeastern Brazil as predator and collector-gatherer. Spies, Froehlich & Kotzian (2006) analyzed larvae in Rio Grande do Sul and Chará-Serna et al. (2010) studied larvae of Colombia, both studies described the genus as shredder. Reynaga (2009), analyzing individuals in a basin of Yungas classified the genus Marilia within the collector-gatherer functional group, while Tomanova et al. (2006) classified it as scraper and collector-gatherer.
The fact that functional assignments are so different in the literature would be associated to the analysis of different species of Marilia, with morphological characteristics of their mouthparts and dissimilar habitat preference. These strategies would allow coexistence of different species without overlapping of their trophic niche.
In this paper, it is suggested that the functional group assignment to the genus level is not recommended for Marilia, and further studies at species level are necessary.
Acknowledgments
We want to thank the researcher group of the IBN (Instituto de Biodiversidad Neo- tropical). This manuscript was supported by Postdoctoral fellowships from CONICET (National Council of Scientific Research, Argentina) and the following grants: PIP- CONICET 11220110100330, PICT-2012-1067 and PICT-2012-2281.
References
Albariño, R. J. & Valverde, A. (1998). Hábito alimenticio del estado larval de Parasericostoma cristatum (Tri-choptera: Sericostomatidae). Revista de la Sociedad Entomológica Argentina, 57, 131-135. [ Links ]
Chará-Serna, A. M., Chará, J. D., Zúñiga, M. C., Pedraza, G. X., & Giraldo, L. P. (2010). Clasificación trófica de insectos acuáticos en ocho quebradas protegidas de la ecorregión cafetera colombiana. Universitas Scientiarum, 15(1), 27-36. [ Links ]
Cheshire, L., Boyero, L., & Pearson, R. G. (2005). Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biology, 50, 748-769. [ Links ]
Cummins, K. W. (1973). Trophic relations of aquatic insects. Annual Review of Entomology, 18, 183-203. [ Links ]
Cummins, K. W., Merritt, R. W., & Andrade, P. C. N. (2005). The use of invertebrates functional group to characterize ecosystem attributes in selected stream and rivers in south Brazil. Studies on Neotropical Fauna and Environment, 40(1), 69-89. [ Links ]
Díaz-Villanueva, V. & Trochine, C. (2005). The role of microorganisms in the diet of Verger cf. limnophilus (Trichoptera: Limnephilidae) larvae in a Patagonian Andean temporary pond. Wetlands, 25(2), 473-479. [ Links ]
Dobson, M., Magana, A., Mathooko, J. M., & Ndegwa, F. K. (2002). Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshwater Biology, 47(5), 909-919. [ Links ]
Henriques-Oliveira, A. L. & Nessimian, J. L. (2010). Spatial distribution and functional feeding groups of aquatic insect communities in Serra da Bocaina streams, southeastern Brazil. Acta Limnologica Brasiliensia, 22(4), 424-441. [ Links ]
Jaksic, F. (2001). Ecología de Comunidades. Santiago de Chile: Ediciones Universidad Católica de Chile. [ Links ]
Martynov, A. B. (1912). Two Collections of Trichoptera from Peru. Annuaire du Musee Zoologique de l’Academie d. Sciences de St. Petersbourg, 17, 1-40. [ Links ]
Merritt, R. W. & Cummins, K. W. (1996). An Introduction to the Aquatic Insects of North America. Iowa: Kendall/Hunt. [ Links ]
Navás, R. P. L. (1931). Insectos de la Argentina, Séptima Serie (1). Revista de la Sociedad Entomológica Argentina, 3, 317-324. [ Links ]
Oliveira, L. G. & Froehlich, C. G. (1997). Diversity and community structure of aquatic insects (Ephemeroptera, Plecoptera and Trichoptera) in a southeastern Brazilian mountain stream. Acta Limnologica Brasiliensia, 9, 139-148. [ Links ]
Peckarsky, B. L. (1996). Predatorprey interactions. In F. R. Hauer & G. A. Lamberti (Eds.), Methods in Stream Ecology (pp. 431-451). California: Academic Press. [ Links ]
Posada-García, J. A. & Roldán-Pérez, G. (2003). Clave ilustrada y diversidad de las larvas de Trichoptera en el Nor-Occidente de Colombia. Caldasia, 25(1), 169-192. [ Links ]
R Development Core Team. (2012). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [ Links ]
Reynaga, M. C. (2009). Hábitos alimentarios de larvas de Trichoptera (Insecta) de una cuenca subtropical. Ecología Austral, 19(3), 207-214. [ Links ]
Reynaga, M. C. & Rueda-Martín, P. A. (2010). Trophic analysis of two species of Atopsyche (Trichoptera: Hydrobiosidae). Limnologica, 40(1), 61-66. [ Links ]
Rincón, J. & Martínez, I. (2006). Food quality and feeding preference of Phylloicus sp. (Trichoptera: Calamoceratidae). Journal of the North American Benthological Society, 25(1), 209-215. [ Links ]
Rueda-Martín, P. A. (2008). Morfología y biología de los estados inmaduros de Marilia cinerea, Navás (1931) y M. elongata, Martynov (1912), con redescripción del macho adulto de M. cinerea (Trichoptera: Odontoceridae). Revista de la Sociedad Entomológica Argentina, 67(1-2), 11-20. [ Links ]
Schoener, T. W. (1970). Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology, 51(3), 408-418. [ Links ]
Spies, M. R., Froehlich, C. G., & Kotzian, C. B. (2006). Composition and diversity of Trichoptera Kirby, 1813 (Insecta) larvae communities in Jacuí River middle section and some tributaries, Rio Grande do Sul State, Brazil. Iheringia Série Zoologia, 96(4), 389-398. [ Links ]
Tomanova, S., Goitia, E., & Helešic, J. (2006). Trophic levels and functional feeding groups of macroin-vertebrates in neotropical streams. Hydrobiologia, 556(1), 251-264. [ Links ]
Ulmer, G. (1905). Neue und wenig bekannte aussereuro-päischer Trichopteren, hauptsächlich aus dem Wiener Museum.Annalen Des K. K. Naturhistorischen Hofs- museums Wien, 20, 59-98. [ Links ]
Wiggins, G. B. (2004). Caddisflies. The Underwater Architects. University of Toronto Press, Toronto. [ Links ]
Chará-Serna, A. M., Chará, J. D., Zúñiga, M. C., Pedraza, G. X., & Giraldo, L. P. (2010). Clasificación trófica de insectos acuáticos en ocho quebradas protegidas de la ecorregión cafetera colombiana. Universitas Scientiarum, 15(1), 27-36. [ Links ]
Cheshire, L., Boyero, L., & Pearson, R. G. (2005). Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biology, 50, 748-769. [ Links ]
Cummins, K. W. (1973). Trophic relations of aquatic insects. Annual Review of Entomology, 18, 183-203. [ Links ]
Cummins, K. W., Merritt, R. W., & Andrade, P. C. N. (2005). The use of invertebrates functional group to characterize ecosystem attributes in selected stream and rivers in south Brazil. Studies on Neotropical Fauna and Environment, 40(1), 69-89. [ Links ]
Díaz-Villanueva, V. & Trochine, C. (2005). The role of microorganisms in the diet of Verger cf. limnophilus (Trichoptera: Limnephilidae) larvae in a Patagonian Andean temporary pond. Wetlands, 25(2), 473-479. [ Links ]
Dobson, M., Magana, A., Mathooko, J. M., & Ndegwa, F. K. (2002). Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshwater Biology, 47(5), 909-919. [ Links ]
Henriques-Oliveira, A. L. & Nessimian, J. L. (2010). Spatial distribution and functional feeding groups of aquatic insect communities in Serra da Bocaina streams, southeastern Brazil. Acta Limnologica Brasiliensia, 22(4), 424-441. [ Links ]
Jaksic, F. (2001). Ecología de Comunidades. Santiago de Chile: Ediciones Universidad Católica de Chile. [ Links ]
Martynov, A. B. (1912). Two Collections of Trichoptera from Peru. Annuaire du Musee Zoologique de l’Academie d. Sciences de St. Petersbourg, 17, 1-40. [ Links ]
Merritt, R. W. & Cummins, K. W. (1996). An Introduction to the Aquatic Insects of North America. Iowa: Kendall/Hunt. [ Links ]
Navás, R. P. L. (1931). Insectos de la Argentina, Séptima Serie (1). Revista de la Sociedad Entomológica Argentina, 3, 317-324. [ Links ]
Oliveira, L. G. & Froehlich, C. G. (1997). Diversity and community structure of aquatic insects (Ephemeroptera, Plecoptera and Trichoptera) in a southeastern Brazilian mountain stream. Acta Limnologica Brasiliensia, 9, 139-148. [ Links ]
Peckarsky, B. L. (1996). Predatorprey interactions. In F. R. Hauer & G. A. Lamberti (Eds.), Methods in Stream Ecology (pp. 431-451). California: Academic Press. [ Links ]
Posada-García, J. A. & Roldán-Pérez, G. (2003). Clave ilustrada y diversidad de las larvas de Trichoptera en el Nor-Occidente de Colombia. Caldasia, 25(1), 169-192. [ Links ]
R Development Core Team. (2012). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [ Links ]
Reynaga, M. C. (2009). Hábitos alimentarios de larvas de Trichoptera (Insecta) de una cuenca subtropical. Ecología Austral, 19(3), 207-214. [ Links ]
Reynaga, M. C. & Rueda-Martín, P. A. (2010). Trophic analysis of two species of Atopsyche (Trichoptera: Hydrobiosidae). Limnologica, 40(1), 61-66. [ Links ]
Rincón, J. & Martínez, I. (2006). Food quality and feeding preference of Phylloicus sp. (Trichoptera: Calamoceratidae). Journal of the North American Benthological Society, 25(1), 209-215. [ Links ]
Rueda-Martín, P. A. (2008). Morfología y biología de los estados inmaduros de Marilia cinerea, Navás (1931) y M. elongata, Martynov (1912), con redescripción del macho adulto de M. cinerea (Trichoptera: Odontoceridae). Revista de la Sociedad Entomológica Argentina, 67(1-2), 11-20. [ Links ]
Schoener, T. W. (1970). Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology, 51(3), 408-418. [ Links ]
Spies, M. R., Froehlich, C. G., & Kotzian, C. B. (2006). Composition and diversity of Trichoptera Kirby, 1813 (Insecta) larvae communities in Jacuí River middle section and some tributaries, Rio Grande do Sul State, Brazil. Iheringia Série Zoologia, 96(4), 389-398. [ Links ]
Tomanova, S., Goitia, E., & Helešic, J. (2006). Trophic levels and functional feeding groups of macroin-vertebrates in neotropical streams. Hydrobiologia, 556(1), 251-264. [ Links ]
Ulmer, G. (1905). Neue und wenig bekannte aussereuro-päischer Trichopteren, hauptsächlich aus dem Wiener Museum.Annalen Des K. K. Naturhistorischen Hofs- museums Wien, 20, 59-98. [ Links ]
Wiggins, G. B. (2004). Caddisflies. The Underwater Architects. University of Toronto Press, Toronto. [ Links ]
*Correspondencia a:
María Celina Reynaga: CONICET, Instituto de Biodiversidad Neotropical (IBN), Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Tucumán, Argentina; celina_reynaga@yahoo.com
Paola Alejandra Rueda Martín: CONICET, Instituto de Biodiversidad Neotropical (IBN), Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Tucumán, Argentina; paolamartinzoo@yahoo.com.ar
1.CONICET, Instituto de Biodiversidad Neotropical (IBN), Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Tucumán, Argentina; celina_reynaga@yahoo.com, paolamartinzoo@yahoo.com.ar
Received 05-VI-2013. Corrected 10-X-2013. Accepted 15-XI-2013.












 uBio
uBio 

