Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.61 n.3 San José Sep. 2013
Growth and photosynthetic performance of five tree seedlings species in response to natural light regimes from the Central Pacific of Costa Rica
Crecimiento y desempeño fotosintético de cinco plántulas de especies arbóreas en respuesta a regímenes lumínicos naturales del Pacífico Central de Costa Rica
Crecimiento y desempeño fotosintético de cinco plántulas de especies arbóreas en respuesta a regímenes lumínicos naturales del Pacífico Central de Costa Rica
*Dirección para correspondencia:
Abstract
Environmental heterogeneity mostly dominated by differing light regimes affects the expression of phenotypic plasticity, which is important for plant growth and survival, especially in the forest understory. The knowledge about these responses to this heterogeneity is a key factor for forest restoration initiatives. In this study, we determine several phenotypic responses to contrasting light conditions in five native tree seedling species of La Cangreja National Park, Central Pacific of Costa Rica, four of them with threatened or relict populations. After 14 weeks at a medium gap condition (24% of full sun), seedlings were transferred and acclimated for 11 weeks to three different natural light regimes: large gap (LG), medium gap (MG) and small gap (SG), corresponding to 52%, 24%, 9% of the mean direct and indirect radiation at each site from full sun. Growth, biomass allocation and leaf gas exchange were measured after the acclimation period. Four species strongly reduced relative growth rate (RGR) in the lower light condition. Total biomass (TB) and RGR were different in Hymenaea courbaril and Platymiscium curiense. H. courbaril and Astronium graveolens had significant changes in the maximum assimilation rate, with a mean value in the LG of 11.02 and 7.70µmolCO2/m2s, respectively. P. curuense showed the same trend and significant changes in RGR and biomass allocation. Aspidosperma myristicifolium and Plinia puriscalensis showed no adjustments to the light regimes in any of the measured variables. This study remarks the importance of determining the growth and physiological performance of these tree native species. It also demonstrates that the most threatened species are those with the less plastic responses to the light regimes, which stresses the difficult situation of their natural populations. This study highlights an urgent definition of the conservation and restoration needs of the degraded forests of the Costa Rican Central Pacific area, where these species dwell.
Key words: assimilation rate, biomass allocation, relative growth rate, light curve responses, phenotypic plasticity, native tree species.
Resumen
La heterogeneidad ambiental dominada mayormente por diferencias en los regímenes lumínicos afecta la expresión de la plasticidad fenotípica, la cual es importante para el crecimiento y la supervivencia de las plantas, especialmente en el sotobosque. Conocer dichas respuestas ante la heterogeneidad es un factor clave para las iniciativas de restauración forestal. En este estudio, determinamos varias respuestas fenotípicas ante condiciones lumínicas contrastantes de cinco especies de plántulas de árboles nativos del Pacífico Central de Costa Rica, algunos de ellos con poblaciones amenazadas. El crecimiento, la asignación de biomasa y el intercambio gaseoso a nivel foliar se midieron al final de once semanas de aclimatación en tres regímenes con diferente radiación: claro grande (LG), claro mediano (MG) y claro pequeño (SG) que corresponden al 54, 24 y 9% de la media del factor de radiación indirecta e indirecta en cada sitio a pleno sol, respectivamente. Cuatro especies presentan fuertes disminuciones en la tasa de crecimiento relativo (RGR) en la condición de poca luz. La biomasa total, RGR y la relación raíz/tallo fueron diferentes para todas las especies. Hymenaea courbaril y Astronium graveolens respondieron significativamente en la tasa de asimilación máxima, con un valor promedio en el LG de 11.02 y 7.70µmolCO2/m2s1 respectivamente. Platymiscium curuense mostró tendencias similares y cambios significativos en la RGR y la asignación de biomasa. Aspidosperma myristicifolium y Plinia puriscalensis mostraron una plasticidad muy baja debido que no expresaron ajustes en ninguna de las variables medidas ante los regímenes de luz. Este estudio resalta la importancia de determinar el crecimiento y el rendimiento fisiológico de estas especies de árboles nativos. También demuestra que las especies más amenazadas son aquellas con las respuestas con menor plasticidad ante los regímenes luz, lo que acentúa la difícil situación de sus poblaciones naturales. Este estudio destaca una urgente definición de las necesidades de conservación y restauración de los bosques degradados de zona del Pacífico Central costarricense, donde estas especies habitan.
Palabras clave: tasa de asimilación, asignación de biomasa, tasa de crecimiento relativo, curva de respuesta a la luz, plasticidad fenotípica, árboles nativos.
Tropical forests present great variability in light regimes (Chazdon & Fetcher 1984, Chazdon & Pearcy 1991), which have influenced the growth and development patterns of many seedlings (Théry 2001), which has been considered a potentially important mechanism to promote local plant biodiversity (Kitajima & Pooter 2008). As sessile organisms, plants have developed a series of morphological and physiological adaptations to cope with environmental light heterogeneity. Changes in these traits may possess a diversity of plastic phenotypic responses in each species (Valladares et al. 2007, Lambers et al. 2008), enabling them to survive in different environments according to their light requirements. Many researchers have looked at the response of native trees in Costa Rica to changes in the light environment (Fournier 1985, Fetcher et al. 1987, Torres & Luján 1999). However, we still have large gaps in scientific and technical knowledge of the light preferences of many species, especially those species with restricted distributional ranges or affected by anthropogenic changes in the original habitats. This information is required, because the recovery of native forests must concentrate on the restoration through an accurate knowledge of the micro-environmental needs and tree performance for the use in forestry.
Some authors have classified plants according to their light requirements (Bazzaz & Pickett 1980, Lambers et al. 2008) as shade tolerants or intolerants (Reich et al. 2003, Valladares & Niinemets 2008). Functional groups studies show that shade intolerants have high phenotypic plasticity (Valladares et al. 2000) than shade tolerant plants in response to the different light regimes. In addition, phenotypic responses in intolerant plants have often been linked to physiological and leaf morphological changes, while tolerant plants seems to respond mostly with architectural traits (e.i. in the branch bifurcation ratio) (Valladares & Niinemets 2008).
This study aims to characterize the growth, biomass allocation and carbon assimilation of seedlings of five tree species native to the Central Pacific area of Costa Rica. This region is dominated by wet forests with a significant dry season which has been severely degraded since last century. Because the selected species have actually reduced populations in their native habitats, our data will facilitate the definition of alternatives to choose better management practices for their reforestation success.
Material and Methodos
Study site: The study was conducted from May through November 2010 at La Cangreja National Park (PNLC), Puriscal, Costa Rica (9º42’10.77’’N-84º23’52.47’’W). This park contains the last remaining patches of tropical wet and premontane wet forest in the Central Pacific region of Costa Rica (Holdridge 1967). The PNLC has a high average annual precipitation (4 000mm), with a rainy season from April to December, high humidity, nutrient-poor soils and variable topography, resulting in great diversity and plant endemism, representing about 7.25% of the country endemic plants (Acosta 1998, Bermúdez 2005).
Study species: We selected three shade-intolerant and two shade-tolerant species native to the Costa Rican Central Pacific region. The Instituto Costarricence de Electricidad (ICE) has a reforestation program in this area and maintains a nursery in the PNLC, and our tree species were obtained from the available stock, reason why chosen plants were in different developmental stage (Table 1). Seedlings of Astronium graveolens, Hymenaea courbaril and Platymiscium curuense were three, four, and six months old, respectively, and were transplanted from the sand beds in the ICE nursery were they germinated. Aspidosperma myristicifolium and Plinia puriscalensis were 12 months old, and were growing in 3-liter pots with soil taken from field sites. These plants were germinated in small pots at conditions similar to our MG light regime. Detailed knowledge about their specific growth and photosynthetic traits is little, but their habitats preferences and distribution is well documented (Table 1). The small number of seedlings obtained from the highly threatened species P. puriscalensis did not allowed us to quantify its biomass allocation, and only gas exchange data are presented for this species. There was no need to artificially watering the seedlings during the experiment because the entire growing period was developed during the rainy season.
Experimental design: We used 30 to 40 seedlings per species and transplanted them to 3.5-litter pots containing a 3:1 mixture of forest topsoil and river sand. Afterwards, seedlings were put under a natural understory light condition, where they were maintained for 14 weeks (first growth period). We harvest 10 plants at the end of the first period which data were only used to calculate the relative growth rate (as explained below). After that first growth period at low light conditions, six to 10 remaining plants per species were transferred to the following three contrasting natural light conditions: large gap (LG), medium gap (MG) and small gap (SG) (Table 2), for a second growth period of 11 weeks. Seedlings were fertilized with 100mL of a complete commercial mineral nutrient formula in the middle of each growing period. The natural light regime of the three sites was characterized by taking eight hemispherical photographs per site, positioned at one meter above the ground using a digital camera (Sigma Inc) in a horizontal position with a 180º fisheye lens (Sigma Inc) coupled with a tripod. These photographs were analyzed with the software HemiView 2.1 (Delta-T Devices Ltd Inc, USA), which considers the location with respect to the magnetic pole, magnetic declination, geographic location and elevation of the site for calculating among others. The following parameters were considered: the direct site factor (DSF), the diffuse site factor (ISF), global site factor (GSF) and leaf area index (LAI), DSF and ISF reflect the proportion of direct and diffuse light, GSF reflects the proportion of canopy gaps and the LAI is an estimation of the leaf area by square meter of ground (Pierce & Running 1988).
Growth and biomass allocation: After each growing period, six to 10 plants per species were harvested to measure total (TB), root (R) and shoot (S) dry biomass, total leaf fresh area (LA), total leaf biomass (LB). Root to shoot ratio (R/S) and specific leaf area (SLA, as LA/LB) were calculated following Beadle (1985). LA was measured with a leaf area meter (Li-3100, LICOR Inc., USA). Total plant bio-mass of the second growth period and the average value of the total biomass of 10 plants of the first growing period were used to calculate relative growth rate (RGR) per plant according to Villar et al. (2004). No root constriction was observed during plant harvest.
Gas exchange measurements: Two open portable photosynthesis systems (LI-6400XT, LICOR, Inc USA and ADC, Inc. London, England) were used to build photosynthetic light response curves for three plants in the second growth, using the following standard conditions. The CO2 ambient concentration was constant during each measurement, but ambient concentration varied from 360 to 390 between measurements. Leaf temperature and relative humidity were maintained around site mean values (25ºC and 60-80%, respectively). We used fully developed and recently expanded leaves produced during the second period, that were pre-illuminated at 1 000-1 200µmol/m2s of photon flux density (PDF) for at least 2min. However, each plant remained 20-60min at ambient light under the mild shade of a plastic canopy before introducing the leaf into the leaf chamber. Carbon assimilation was measured at several PDF values ranging from 1 500 to 0µmol/m2s from a red light source (LICOR Part 6400-02 LED, 660 -675nm wavelength range and the ADC Light units LED’s, 660nm wavelength and between 5-10% of blue light) attached to the leaf chamber. Assimilation rates versus photosynthetic active radiation data were fitted to the following empirical model (Küppers & Schulze 1985):
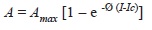
where A is the rate of carbon assimilation, Amax is light-saturated rate of photosynthesis, Ø is a parameter that describes the shape of the light response curve apparent quantum yield, I is irradiance and corresponds to the incident PDF (µmol/m2s) and Ic is the light compensation point. The light response curves were fitted by SigmaPlot 11.0 curve fitting (Systat Software, Inc., California) to obtain the parameters Amax and Ø. Independently, the slope of a linear regression of A values between 0 and 50µmol/ m2s of PDF was used to calculate the apparent quantum yield (Qapp). Dark respiration (Rd) was calculated from the intersection on the “y” axis, and the intersection of “x” axis was used to obtain Ic.
All parameters measured at the end of the second period were compared using a one way ANOVA and a posteriori Tukey tests mean comparison with the goal of evidence differences between exposure treatments. A principal components analysis (PCA) per species was done on growth and biomass allocation parameters to show what combination of parameters explains most of the total variance. The component scores extracted from the first two principal components were tested by an analysis of variance comparing them by exposure treatments. All analysis were made following Quinn & Keough (2002), using the software JMP 7 (SAS).
Results
Light environments: Based on the GSF our three sites represented conditions of light gaps of approximately 50, 25 and 10% of full sunlight (Table 2). LAI was similar between the large (LG) and the medium light gap (MG), but both of these places had a significant low LAI respect to the small light gap (SG) condition. The LG condition received 25 and 40% more of the ISF and DSF than the MG and SG conditions, respectively. These results show that the sites used for the light treatment acclimation were significantly contrasting between them, and represent a diversity of light regimes naturally found in the present conditions of the forested areas of the Central Pacific zone.
Growth and biomass allocation: The four species presented a gradient of mean SLA in the following order: P. curuense, A. graveolens, H. courbaril and A. myristicifolium. None of the species showed significant changes in SLA between light conditions (Table 3). Root to shoot ratio was larger in H. courbaril and A. myristicifolium (0.517 and 0.496 respectably) and smaller in A. graveolens (0.238), but none of this species adjusted the R/S according to the prevailing light treatment. Only P. curuense showed a significant lower mean of R/S in the LG treatment compared to the other two light growing conditions. However, this is contrary to our expectations. Total biomass was significantly lower in the SG treatment for both H. courbaril and P. curuense, and the same trend was observed in A. myrsticifolium. However, this species and A. graveolens did not changed significantly in biomass between light treatments.
P. curuense and A. graveolens showed the larger variation in RGR (Fig. 1), being the species with the larger and smaller plants, respectively at the beginning of the experiment. These species were significantly affected by the light regime (H. courbaril: F2/23= 12.52, p< 0.001, and P. curuense: F 2/23= 4.01, p=0.03). The species A. graveolens had the higher RGR value, and it was the only species with a positive RGR in the forest understory conditions. A. myristicifolium presented lower RGR values across the three light regimes, and they did not differ between them (Fig. 1).
The PCA’s for the growth and biomass allocation variables per species (Fig. 2) show that the first and the second component explained in all species more than 50 and 22% of the variation, respectively. In all case (except A. graveolens) the first component is influenced largely by RGR and TB, while the second was mostly influenced by SLA and R/S (Table 4). Analyses of variance of the score values extracted from the PCA’s showed a significant difference between treatments of the first components for H. courbaril and P. curuense (F2/23= 12.06, p<0.001; F2/23= 4.77, p<0.01, respectively) (Fig. 2). The other components of these and the other species did not show differences (p>0.05).
Gas exchange measurements: In general, the light response curve of all species shows a maximum light saturation just above 600µmol/ m2s (Fig. 3). Amax comparisons between species were made at 1 000µmol/m2s values to ensure the maximum leaf photosynthetic activity. The light response curves clearly changed in the shade intolerant species and remained similar between light treatments in the two shade tolerant species (Fig. 3).
The shade-intolerant species had maximum assimilation rates in agreement with the present light environments in which they developed (Fig. 4), that is, higher Amax in the LG treatment than in the SG treatment. These trends among treatments were only significantly different in H. courbaril (F2/6= 5.89, p= 0.02) and A. graveolens (F2/4= 7.84, p= 0.04). The other two shade-tolerant species did not change their maximum photosynthetic response to the prevailing light regimes, but they both showed a much lower Amax compared with the shade intolerant species. Of the other variables extracted from light curves response only stomatal conductance in H. courbaril was significantly lower in the SG environment (Table 5). This trend was followed by P. curuense and A. myristicifolium. Mean dark respiration was always higher than -0.88µmolCO2/m2s across species and treatments. There were no significant changes in mean transpiration or water use efficiency between treatments or species (Table 5). There were no changes in apparent quantum yield, which varied between 0.04 and 0.08. Similarly, light compensation points were as low as 4µmol/m2s in H. courbaril at SG, and as high as 16.7µmol/m2s in A. graveolens in the MG treatment.
Discussion
The plasticity to light acclimation is directly related to physiological, morphological and growth traits (Bazzaz & Carlson 1982, King 1991). In this connection, our species can be separated into two groups: (1) high plasticity species (H. courbaril, P. curuense and A. graveolens) and (2) low plasticity species (A. myrsticifolium and P. puriscalensis). The species of these two groups can certainly be categorized as shade intolerant and shade tolerant species according to their light habitat preferences, where the first group is differentiated by the faster growth, greater biomass accumulation, and a clear acclimation response in maximum assimilation rate according to light conditions.
Several studies show shade-tolerant species as usually having low phenotypic plasticity against the light regimes (Poorter 1999, Valladares et al. 2000, Portsmuth & Niinemets 2007), which has been considered a functional trait for the survival and development in environments where the resource is low (Valladares et al. 2007, Valladares & Niinemets 2008). Other traits normally ascribed to shade-tolerant species include lower respiration values, lower maximum assimilation and lower SLA than intolerant species (Kitajima 1994, Reich et al. 2003, Lusk et al. 2011). However, none of these traits showed clear trends between our five studied species.
Depending on the magnitude of the light radiation, the shade-intolerant species may exhibit a number of physiological and morphological adaptations of greater plasticity when they are in low radiation (Rice & Bazzaz 1989, Lambers et al. 2008), such as bifurcations of the branches and horizontal angles in the leaves (Lambers et al. 2008, Valladares & Niinemets 2008). These variables were not measured in this study, although it is possible that they could be key parameters in the acclimation regimes. In general, the five species are a very idiosyncratic selection. For example, if we consider RGR as the only parameter to classify species performance, we must classify A. myristicifolium and H. courbaril as shade-intolerant species, giving its negative RGR at the lower light regime. However, only H. courbaril responded with significant changes of their maximum assimilation rates to the prevailing light conditions. This example explains why it is important to study various parameters for the characterization of performance of the species to its environment and their changes. It is noteworthy to consider that normal understory conditions are about 2-3% of full sun in tropical primary forests (Chazdon & Fetcher 1984), which is significantly lower compared with our low light regime of about 9% of full sunlight. For this reason, we cannot discard that this not so low light condition could reduce the diffe-rences obtained. For example, the SLA is a variable that commonly shows large variation in low and high light levels sites, in temperate and tropical environments (Valladares et al. 2000, Lambers et al. 2008). In this sense, SLA can almost be used as a good indicator of subtle plastic changes in light regimes for many species. This situation confirms that our low light treatment was a little above average for a forest understory.
By reducing resource allocation to bio-mass in general (Poorter 1999, Lambers & Poorter 2004), shade-intolerant plants may experience rapid adaptation to the site where they are (Montgomery & Chazdon 2002). These functional features are probably responsible for differences in RGR, TB and maximum assimilation rates in the shade-intolerant species than shade-tolerant species.
It is clear that the less plastic species, A. myrsticifolium and P. puriscalensis, are the two shade tolerant species with the smaller growth rates and the ones with the most critical conditions in their remnant populations. P. puriscalensis has a reduced population distribution, known only in the PNLC (Jiménez 2001), and A. myristicifolium has restricted distributions in primary and secondary forest (Morales 2001), aspects that should probably put both as endangered species. The present growth and physiological results are intended to help in the decision making about the use of these species, and all other species in similar situation, in restoration and conservation projects, either through the selection of sites with suitable light conditions for the growth and establishment of species or in the selection of environments to protect the natural forest where these species still dwell. We recommend for future reforesta-tion programs to avoid high light regime sites for A. myristicifolium and P. puriscalensis, and to keep its distribution within their natural range (Table 1). H. courbaril, P. curuense and A. graveolens are species that can be planted in open areas after a short acclimation to high light in nurseries when seedlings. Larger sizes can be used directly into reforestation trails along the Pacific slopes. Any effort in this sense will help reduce the speed of biodiversity loss if our degraded tropical areas.
Acknowledgments
We thank the staff of the Parque Nacional La Cangreja and the nursery staff of ICE for provided facilities. Adrian Rodríguez, German Vargas and Gilberth Mora helped with the field data, and to Gerardo Avalos and three anonymous reviewers for suggestions to previous versions. This study was supported by two grants from the Fondo Especial para la Educación Superior through the (Consejo Nacional de Rectores de Costa Rica), one to Roberto Cordero and other equipment grant (Licor-6400XT system) to Escuela de Ciencias Agrarias, both to Universidad Nacional of Costa Rica.
References
Acosta V., L.G. 1998. Análisis de la composición florística y estructura para la vegetación del piso basal de la zona protectora La Cangreja, Mastatal de Puriscal. Informe de Práctica de Especialidad, Instituto Tecnológico de Costa Rica, Cartago, Costa Rica. [ Links ]
Bazzaz, F.A. & R.W. Carlson. 1982. Photosynthetic acclimation to variability in the light environment of early and late successional plant. Oecologia 54: 313-316. [ Links ]
Bazzaz, F.A. & S.T.A. Pickett. 1980. Physiological ecology of tropical succession: a comparative review. Ann. Rev. Ecol. Syst. 11: 287-310. [ Links ]
Beadle, C.L. 1985. Plant growth analysis, p. 20-25. In J. Coombs, D.O. Hall, S.P. Long & J.M.O. Scurlock (eds.). Techniques in Bioproductivity and Photosynthesis. Oxford, USA. [ Links ]
Bermúdez, F.A. 2005. Plan de manejo del Parque Nacional La Cangreja Puriscal. Ministerio del Ambiente y Energía. Puriscal, San José, Costa Rica. [ Links ]
Chazdon, R.L. & N. Fetcher. 1984. Photosynthetic light environments in lowland tropical rain forest in Costa Rica. J. Ecol. 72: 553-564. [ Links ]
Chazdon, R.L. & R.W. Pearcy. 1991. The importance of sunflecks for forest understory plants. BioScience 41: 760-766. [ Links ]
Cordero, J. & D.H. Boshier. 2003. Árboles de Centroamérica: un manual para extensionistas. Centro Agronómico de Investigación y Enseñanza, Turrialba, Costa Rica and Oxford Forestry Institute, United Kingdom. [ Links ]
Fetcher, N., S.F. Oberbauer, G. Rojas & B.R. Strain. 1987. Efectos del régimen de luz sobre la fotosíntesis y el crecimiento en plántulas de árboles de un bosque lluvioso tropical de Costa Rica. Rev. Biol. Trop. 35: 97-110. [ Links ]
Fournier, J. 1985. El sector forestal en Costa Rica: Antecedentes y perspectivas. Agron. Costarr. 9: 253-260. [ Links ]
Holdridge, L.R. 1967. Life Zone Ecology. Tropical Science Center, San José, Costa Rica. [ Links ]
Jiménez, Q. 2001. Plinia puriscalensis (P.E. Sánchez & Q. Jiménez). Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica. (Downloaded: February 11, 2012, http://darnis.inbio.ac.cr/FMPro?-DB=UBIpub.fp3&-lay=WebAll&Format=/ubi/detail.html&-Op=bw&id=4600&-Find). [ Links ]
Jiménez, Q., A. Estrada, A. Rodríguez & P. Arroyo. 1996. Manual dentrológico de Costa Rica. Instituto Tecnológico de Costa Rica, Cartago, Costa Rica. [ Links ]
Jiménez, Q., F.E. Rojas, V. Rojas & L. Rodríguez. 2011. Árboles maderables de Costa Rica: Ecología y silvicultura. Second edition. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. [ Links ]
King, D.A. 1991. Correlations between biomass allocation, relative growth rate and environment in tropical forest sampling. Funct. Ecol. 5: 485-492. [ Links ]
Kitajima, K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419-428. [ Links ]
Kitajima, K. & L. Pooter. 2008. Functional basis for resource niche partitioning by tropical trees, p. 160-181. In. W.P. Carson & S.A. Schnitzer (eds.). Tropical Forest Community Ecology. Blackwell Publishing, Oxford, United Kingdom. [ Links ]
Küppers, M. & E.D. Schulze. 1985. An empirical model of net photosynthesis and leaf conductance for the simulation of diurnal courses of CO2 and H2O exchange. Aust. J. Plant Physiol. 12: 513-526. [ Links ]
Lambers, H. & H. Poorter. 2004. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 34: 283-262. [ Links ]
Lambers, H., F.S. Chapin & T.L. Pons. 2008. Plant physiological ecology. Springer, New York, USA. [ Links ]
Lusk, C.H., M.M. Pérez-Millaqueo, F.I. Piper & A. Saldaña. 2011. Ontogeny, understory light interception and simulated carbon gain of juvenile rainforest evergreens differing in shade tolerance. Ann. Bot. 108: 419-428. [ Links ]
Martin, W.A. & E.M. Flores. 2002. Astronium graveolens Jacq, p. 311-314. In. J.A. Vozzo (ed.). Tropical tree seed manual. United States Department of Agriculture, Forest Service, USA. [ Links ]
Montgomery, R.A. & R.L. Chazdon. 2002. Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia 131: 165-174. [ Links ]
Morales, J.F. 2001. Aspidosperma myristicifolium (Mark-gr.) Woodson. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. (Downloaded: February 11, 2012, http://darnis.inbio.ac.cr/ubis/FMPro?-DB=UBIPUB.fp3&lay=WebAll&-error=norec.html&-Format=detail.html&-Op=eq&id=1835&-Find). [ Links ]
Pierce, L.L. & S.W. Running. 1988. Rapid estimation of coniferous forest leaf area index using a portable integrating radiometer. Ecology 69: 1762-1767. [ Links ]
Poorter, L. 1999. Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct. Ecol. 13: 396-410. [ Links ]
Portsmuth, A. & Ü. Niinemets. 2007. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 21: 61-77. [ Links ]
Quinn, G. & M. Keough. 2002. Experimental design and data analysis for biologists. Cambridge University, New York, USA. [ Links ]
Reich, P.B., I.J. Wright, J. Cavender-Bares, M. Craine, J. Oleksyn, M. Westoby & M.B. Walters. 2003. The evolution of plant functional variation: traits, spectra and strategies. Int. J. Plant Sci. 164: S143-S164. [ Links ]
Rice, S.A. & F.A. Bazzaz. 1989. Growth consequences of plasticity of plant traits in response to light conditions. Oecologia 78: 508-512. [ Links ]
Sánchez, P.E. & Q. Jiménez. 1989. Una nueva especie de Plinia L. (Myrtaceae) para Costa Rica. Brenesia 32: 113-116. [ Links ]
Théry, M. 2001. Forest light and its influence on habitat selection. Plant Ecol. 157: 251-261. [ Links ]
Torres, G. & F.R. Luján. 1999. Especies forestales nativas con potencial para la reforestación en las regiones Brunca y Pacífico Central de Costa Rica. Boletín Kurú 27: 2-6. [ Links ]
Valladares, F. & Ü. Niinemets. 2008. Shade Tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 39: 237-257. [ Links ]
Valladares, F., E. Gianli & J.M. Gómez. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176: 749-763. [ Links ]
Valladares, F., S.J. Wright, E. Lasso, K. Kitajima & R.W. Pearcy. 2000. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81: 1925-1936. [ Links ]
Villar, R., J. Ruíz-Robleto, J.L. Quero, H. Poorter, F. Valladares & T. Marañón. 2004. Tasas de crecimiento en especies leñosas: aspectos funcionales e implicaciones ecológicas, p. 193-230. In F. Valladares (ed.). Ecología del bosque mediterráneo en un mundo cambiante. EGRAF S. A., Madrid, Spain. [ Links ]
Zamora, N., Q. Jiménez. & L.J. Poveda. 2000. Árboles de Costa Rica, Vol II. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. [ Links ]
Bazzaz, F.A. & R.W. Carlson. 1982. Photosynthetic acclimation to variability in the light environment of early and late successional plant. Oecologia 54: 313-316. [ Links ]
Bazzaz, F.A. & S.T.A. Pickett. 1980. Physiological ecology of tropical succession: a comparative review. Ann. Rev. Ecol. Syst. 11: 287-310. [ Links ]
Beadle, C.L. 1985. Plant growth analysis, p. 20-25. In J. Coombs, D.O. Hall, S.P. Long & J.M.O. Scurlock (eds.). Techniques in Bioproductivity and Photosynthesis. Oxford, USA. [ Links ]
Bermúdez, F.A. 2005. Plan de manejo del Parque Nacional La Cangreja Puriscal. Ministerio del Ambiente y Energía. Puriscal, San José, Costa Rica. [ Links ]
Chazdon, R.L. & N. Fetcher. 1984. Photosynthetic light environments in lowland tropical rain forest in Costa Rica. J. Ecol. 72: 553-564. [ Links ]
Chazdon, R.L. & R.W. Pearcy. 1991. The importance of sunflecks for forest understory plants. BioScience 41: 760-766. [ Links ]
Cordero, J. & D.H. Boshier. 2003. Árboles de Centroamérica: un manual para extensionistas. Centro Agronómico de Investigación y Enseñanza, Turrialba, Costa Rica and Oxford Forestry Institute, United Kingdom. [ Links ]
Fetcher, N., S.F. Oberbauer, G. Rojas & B.R. Strain. 1987. Efectos del régimen de luz sobre la fotosíntesis y el crecimiento en plántulas de árboles de un bosque lluvioso tropical de Costa Rica. Rev. Biol. Trop. 35: 97-110. [ Links ]
Fournier, J. 1985. El sector forestal en Costa Rica: Antecedentes y perspectivas. Agron. Costarr. 9: 253-260. [ Links ]
Holdridge, L.R. 1967. Life Zone Ecology. Tropical Science Center, San José, Costa Rica. [ Links ]
Jiménez, Q. 2001. Plinia puriscalensis (P.E. Sánchez & Q. Jiménez). Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica. (Downloaded: February 11, 2012, http://darnis.inbio.ac.cr/FMPro?-DB=UBIpub.fp3&-lay=WebAll&Format=/ubi/detail.html&-Op=bw&id=4600&-Find). [ Links ]
Jiménez, Q., A. Estrada, A. Rodríguez & P. Arroyo. 1996. Manual dentrológico de Costa Rica. Instituto Tecnológico de Costa Rica, Cartago, Costa Rica. [ Links ]
Jiménez, Q., F.E. Rojas, V. Rojas & L. Rodríguez. 2011. Árboles maderables de Costa Rica: Ecología y silvicultura. Second edition. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. [ Links ]
King, D.A. 1991. Correlations between biomass allocation, relative growth rate and environment in tropical forest sampling. Funct. Ecol. 5: 485-492. [ Links ]
Kitajima, K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419-428. [ Links ]
Kitajima, K. & L. Pooter. 2008. Functional basis for resource niche partitioning by tropical trees, p. 160-181. In. W.P. Carson & S.A. Schnitzer (eds.). Tropical Forest Community Ecology. Blackwell Publishing, Oxford, United Kingdom. [ Links ]
Küppers, M. & E.D. Schulze. 1985. An empirical model of net photosynthesis and leaf conductance for the simulation of diurnal courses of CO2 and H2O exchange. Aust. J. Plant Physiol. 12: 513-526. [ Links ]
Lambers, H. & H. Poorter. 2004. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 34: 283-262. [ Links ]
Lambers, H., F.S. Chapin & T.L. Pons. 2008. Plant physiological ecology. Springer, New York, USA. [ Links ]
Lusk, C.H., M.M. Pérez-Millaqueo, F.I. Piper & A. Saldaña. 2011. Ontogeny, understory light interception and simulated carbon gain of juvenile rainforest evergreens differing in shade tolerance. Ann. Bot. 108: 419-428. [ Links ]
Martin, W.A. & E.M. Flores. 2002. Astronium graveolens Jacq, p. 311-314. In. J.A. Vozzo (ed.). Tropical tree seed manual. United States Department of Agriculture, Forest Service, USA. [ Links ]
Montgomery, R.A. & R.L. Chazdon. 2002. Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia 131: 165-174. [ Links ]
Morales, J.F. 2001. Aspidosperma myristicifolium (Mark-gr.) Woodson. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. (Downloaded: February 11, 2012, http://darnis.inbio.ac.cr/ubis/FMPro?-DB=UBIPUB.fp3&lay=WebAll&-error=norec.html&-Format=detail.html&-Op=eq&id=1835&-Find). [ Links ]
Pierce, L.L. & S.W. Running. 1988. Rapid estimation of coniferous forest leaf area index using a portable integrating radiometer. Ecology 69: 1762-1767. [ Links ]
Poorter, L. 1999. Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct. Ecol. 13: 396-410. [ Links ]
Portsmuth, A. & Ü. Niinemets. 2007. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 21: 61-77. [ Links ]
Quinn, G. & M. Keough. 2002. Experimental design and data analysis for biologists. Cambridge University, New York, USA. [ Links ]
Reich, P.B., I.J. Wright, J. Cavender-Bares, M. Craine, J. Oleksyn, M. Westoby & M.B. Walters. 2003. The evolution of plant functional variation: traits, spectra and strategies. Int. J. Plant Sci. 164: S143-S164. [ Links ]
Rice, S.A. & F.A. Bazzaz. 1989. Growth consequences of plasticity of plant traits in response to light conditions. Oecologia 78: 508-512. [ Links ]
Sánchez, P.E. & Q. Jiménez. 1989. Una nueva especie de Plinia L. (Myrtaceae) para Costa Rica. Brenesia 32: 113-116. [ Links ]
Théry, M. 2001. Forest light and its influence on habitat selection. Plant Ecol. 157: 251-261. [ Links ]
Torres, G. & F.R. Luján. 1999. Especies forestales nativas con potencial para la reforestación en las regiones Brunca y Pacífico Central de Costa Rica. Boletín Kurú 27: 2-6. [ Links ]
Valladares, F. & Ü. Niinemets. 2008. Shade Tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 39: 237-257. [ Links ]
Valladares, F., E. Gianli & J.M. Gómez. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176: 749-763. [ Links ]
Valladares, F., S.J. Wright, E. Lasso, K. Kitajima & R.W. Pearcy. 2000. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81: 1925-1936. [ Links ]
Villar, R., J. Ruíz-Robleto, J.L. Quero, H. Poorter, F. Valladares & T. Marañón. 2004. Tasas de crecimiento en especies leñosas: aspectos funcionales e implicaciones ecológicas, p. 193-230. In F. Valladares (ed.). Ecología del bosque mediterráneo en un mundo cambiante. EGRAF S. A., Madrid, Spain. [ Links ]
Zamora, N., Q. Jiménez. & L.J. Poveda. 2000. Árboles de Costa Rica, Vol II. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Heredia, Costa Rica. [ Links ]
*Correspondencia a:
J. Antonio Guzmán Q.. Laboratorio de Ecología Vegetal Funcional (LEVEF), Escuela de Ciencias Biológicas, Universidad Nacional de Costa Rica, Campus Omar Dengo, Heredia, Costa Rica; antguz06@gmail.com
Roberto A. Cordero S.. Laboratorio de Ecología Vegetal Funcional (LEVEF), Escuela de Ciencias Biológicas, Universidad Nacional de Costa Rica, Campus Omar Dengo, Heredia, Costa Rica; ticolamb@gmail.com
1. Laboratorio de Ecología Vegetal Funcional (LEVEF), Escuela de Ciencias Biológicas, Universidad Nacional de Costa Rica, Campus Omar Dengo, Heredia, Costa Rica; antguz06@gmail.com, ticolamb@gmail.com
Received 16-VII-2012. Corrected 10-XII-2012. Accepted 24-I-2013.














