Revista de Biología Tropical
versión On-line ISSN 0034-7744versión impresa ISSN 0034-7744
Rev. biol. trop vol.61 no.3 San José sep. 2013
Three new species of Hemibrycon (Characiformes: Characidae) from the Magdalena River Basin, Colombia
Tres nuevas especies de Hemibrycon (Characiformes: Characidae) de la cuenca del río Magdalena, Colombia
Tres nuevas especies de Hemibrycon (Characiformes: Characidae) de la cuenca del río Magdalena, Colombia
César Román-Valencia1*, Raquel I. Ruiz-C.1, Donald C. Taphorn3*, Néstor J. Mancera-Rodriguez2* & Carlos A. García-Alzate1,4*
*Dirección para correspondencia:
Abstract
Fish biodiversity of aquatic ecosystems is highly threatened by different economic activities driven by human populations, and its description is increasingly a priority. For the Cauca-Magdalena River system we have described 14 species, and the purpose of this paper was to describe three new species belonging to the same genus Hemibrycon from the Nare and Guatapé River drainages of the middle Magdalena River, Colombia. The description was based on a series of 200 specimens, and the use of morphometric, meristic and osteological characters, as well as fish distribution and morphogeometric analytical methods. We have found that Hemibrycon fasciatus n. sp. (n=54) differs from other species of Hemibrycon (that also have a vertical humeral spot) in having: melanophores outlining the posterior margins of the scales along sides of body; humeral spot extending onto posterior margin of opercle; a dark lateral stripe, formed by deep pigment that is continuous with the peduncular spot; the toothed portion of the maxilla not reaching the dorsal margin of the dentary (vs. toothed portion of maxilla extending beyond dorsal margin of dentary); all maxillary teeth tricuspid (vs. some unicuspid teeth present on maxilla). H. cardalensis n. sp. (n=64) differs in having: a vertically elongate humeral spot that extends one or two scales below the lateral line canal. H. cardalensis n. sp. differs from all congeners in having the pigment of the caudal spot restricted to the ventral half of the caudal peduncle, and in having melanophores around the anterior scales of the lateral line. Hemibrycon antioquiae n. sp. (n=82) differs in having a circular humeral spot. It differs from the other species with a circular humeral spot, like H. mikrostiktos, in having a projection of disperse melanophores extending from the dorsal margin of the humeral spot to below the lateral stripe. Habitat data and environmental impacts caused by the construction of reservoirs for hydroelectric projects and other threats in the area are included, as well as a key to all species Hemibrycon present in the Magdalena River Basin. The synonymy of H. pautensis with H. polyodon is discussed and H. pautensis is evalidated.
Key words: tropical fish, new taxon, scales, morphology, pigmentation pattern.
Resumen
Un total de 14 especies del género Hemibrycon Günther se han encontrado en la cuenca del río Magdalena- Cauca de Colombia. En este estudio, se describen tres nuevas especies de peces del género Hemibrycon Günther 1864 (Characiformes: Characidae) de la cuenca del río Magdalena con una serie de 200 ejemplares, con caracteres morfométricos, merísticos, osteológicos y distribución, y bajo un método de análisis morfogeométrico: Hemibrycon fasciatus n. sp. (n=54), se distingue de otras especies de Hemibrycon por una mancha humeral vertical y por presentar: melanóforos sobre el borde posterior de las escamas a lo largo de la parte lateral del cuerpo; la mancha humeral extendida sobre el borde posterior del opérculo; una banda lateral oscura, formada por pigmentos profundos que se prolongan con la mancha peduncular; la longitud del maxilar con dientes que no alcanzan el borde dorsal del dentarío (vs. longitud con dientes del maxilar sobrepasan el borde dorsal del dentarío); todos los dientes del maxilar tricúspides (vs. dientes unicúspides en el maxilar). H. cardalensis n. sp. (n=64) se distingue de todos sus congéneres por presentar los pigmentos del pedúnculo caudal restringidos a su mitad ventral, y por exhibir melanóforos alrededor de las escamas anteriores del canal latero sensorial. Hemibrycon antioquiae n. sp. (n=82) se distingue en el área predorsal que es sobresaliente respecto a la superficie dorsal del cráneo, por una banda lateral oscura, y por la forma de la mancha peduncular que es ancha y se extiende sobre la banda lateral (vs. banda lateral plateada sin melanóforos y mancha peduncular no conspicua). Se distingue de todos sus congéneres por la forma rectangular del borde posterior del cleitro. Se incluyen datos del hábitat, los efectos antrópicos generados por la construcción de embalses o represas para proyectos hidroeléctricos en el hábitat propio de las especies y una clave taxonómica de las especies de Hemibrycon presentes del río Magdalena. También se discute la sinonimia de H. pautensis con H. polyodon y por lo tanto se revalida H. pautensis.
Palabras clave: pez tropical, nuevo taxon, escamas, morfología, patrón de pigmentación.
The taxonomy of the genus Hemibrycon Günther has been fairly addressed (Román-Valencia et al. 2006, 2007, 2009a, 2009b, Román-Valencia & Ruiz 2007, Bertaco et al. 2007, Román-Valencia & Arcila-Mesa 2008, 2009, 2010, Bertaco & Malabarba 2010). A total of 14 species have been found in the Cauca-Magdalena River Basin of Colombia, including the three described here in: H. boquiae, H. brevispini, H. cairoense, H. colombianus, H. palomae, H. paez, H. quindos, H. rafaelense, H. raqueliae, H. virolinica, and H. yacopiae (Román-Valencia et al. 2006, 2007, 2009a, 2009b, 2010, Román-Valencia & Ruiz-C. 2007, Román-Valencia & Arcila-Mesa 2008, 2009, 2010). The increase in the number of described Hemibrycon species from the Cauca-Magdalena River system, most of which exist in allopatry, suggests an interesting model for diversification of this genus in the Andes (Román-Valencia & Arcila-Mesa 2009).
Many Andean fishes are threatened by human developments and this is the case for these new species, because their habitat is imperiled by the construction and operation of hydroelectric dams, and other factors. The purpose of this paper is to describe three new species of Hemibrycon from the Nare and Guatapé River drainages of the middle Magdalena River, as a further contribution of the first author’s ongoing revision of Hemibrycon species in South America; yet more proof of the undocumented biodiversity of the genus.
Materials and Methods
Cast nets and electro fishing gear were used to capture these fishes from nine creeks in fiv areas of the middle reaches of the Nare River, above the Peñol-Guatapé dam, and between that site and the San Lorenzo reservoir, and the Guatapé River drainage, and both above and below the Playas reservoir. This region includes parts of the following counties (Municipios): Alejandría, Concepción, Guatapé, San Vicente and San Rafael, in the state (Departamento) of Antioquia, Colombia (Fig. 1), that will be described in the next paragraphs.
Zone 1: Sampling stations were located in the Nare River drainage above the Peñol-Guatapé reservoir. Collections were made in La Compañía and La Magdalena Creeks, at altitudes between 1 875 and 2 094m.a.s.l, and in different dates (November 2007, March and May 2008, January and March 2010, June, September and November 2011, and February 2012). These sites had major human impacts, with obvious contamination, bad odors, garbage, sewage and agricultural runoff from adjacent fields.
• La Compañía Creek: Located in San Vicente County, this stream receives sewage from much of the region and empties into the Nare River above the Peñol-Guatapé reservoir. The sampling site was at 2 094m.a.s.l. The substrate was sandy, with medium sized rocks; water was dark because of suspended organic material. Depth was 1.0-1.80m.
• La Magdalena Creek: Located in San Vicente County, this site had better water quality than La Compañía Creek. Sampling sites were located between 1 875 and 2 140m.a.s.l. Water was clear over a sandy to rocky substrate; the sampling sites were near a path through cattle pastures and agricultural fields where beans, strawberries, and sisal crops were grown. Local inhabitants informed us that the creek was affected when the fibers from the sisal plants are washed after harvest, and also, by fertilizers and pesticides. Sewage pipes flowing into streams from several houses were also observed in the area.
Zone 2: These sites were located in the Nare River drainage below the Peñol-Guatapé reservoir:
• Santa Gertrudis Creek: Located below the outflow chute of the Peñol-Guatapé Reservoir at an altitude of 1 820m.a.s.l. substrate was rocky, water clear to crystalline and the stream was affected by the discharge from the reservoir. Water quality was better than that in creeks of Zone 1, with rapid flow and good oxygenation.
Zone 3: Stations located in the Nare River drainage above the San Lorenzo reservoir:
• San Lorenzo and San José Creeks: Located between 1 250 and 1 261m.a.s.l. respectively, these creeks empty directly in to the San Lorenzo Reservoir. In this sector there are very large rocks, but upstream the substrate is sandy. Fish capture was easier in areas where loose rocks served as refuges. Depth of San Lorenzo Creek was between 0.4-1.50m and at sites in San José Creek, from 0.20-0.70m.
Zone 4: Sites located in the Guatapé River drainage, above the Playas Reservoir.
• Guatapé River drainage, Peñoles and Mazorcos Creeks: these are located in San Rafael County, substrates were rocky and slopes were steep. Sites were located between 1 201 and 1 265m.a.s.l. The region was partially forested with native vegetation, but cattle pastures were also present. Very large rocks were present in and around the stream.
Zone 5: These sites were located in the Guatapé River drainage below Playas Reservoir.
• El Cardal Creek: This stream is located in the Guatapé River drainage, below Playas Reservoir. Substrate was sandy to rocky, with very large rocks upstream from the sampling station. Water was moderately contaminated. At some spots in this creek, decomposing organic material caused bad odors. Mining activity was also present, but both sides of the channel had native vegetation. In the upper section of this creek, sediment deposits were noted, along with very large rocks, with lichens growing on the exposed portions. El Cardal Creek near the two-lane bridge on the highway between San Rafael and San Carlos counties receives the discharge of sewage pipes coming from a housing development of the “Empresas Públicas de Medellín” (EPM) company.
Measurements were taken with digital calipers, recorded to hundredths of millimeters and usually expressed as percentages of standard or head length (Table 1). Counts were made using a stereoscope with a dissection needle to extend the fins. Counts and measurements were taken from the left side of specimens when possible and in general were taken according to guidelines in Vari & Siebert (1990).
Observations of bones and cartilage were made using cleared and stained specimens (C & S), prepared according to techniques outlined in Taylor & Van Dyke (1985) and Song & Parenti (1995). Bone nomenclature follows Weitzman (1962), Vari (1995) and Ruiz-C. & Román-Valencia (2006). Museum acronyms follow Sabaj-Perez (2012).In the list of paratypes, the number of individuals is given immediately after the catalog number, which is followed by the range of Standard Length (SL) in mm for that lot. All collections were made in Colombia, Antioquia state (department), from the Nare and Guatapé Rivers Basins. Some locality information is not translated because key information from original labels is often lost by inaccurate translation. The holotypes were kept in the laboratory of Ichthyology, Universidad del Quindío, Armenia, Colombia (IUQ).
The 21 morphometric characters analyzed in this study (Table 1) were evaluated by Principal Component Analysis (PCA) using the Burnaby method to eliminate the influence of size with the PAST program, session 2.13 for Windows (Hammer et. al. 2008). Also, a morphogeometric analysis was done to discriminate populations from the Nare and Guatapé River drainages (Fig. 1) that coincide with the three new species described here. For that analysis a digital image was taken of the left side of each individual using a CyberShot w360 Sony digital camera installed on a photography stand. The macro lens option was used to reduce deformation of the edges of the images. Millimeter graph paper was used as a background for these images to determine if deformation was occurring, and the scale of each image (Ruiz-C. & Cipriani 2006, García-Alzate et al. 2011, Ruiz-C. et al. 2011). TPS files were created using the software program TPSUtil (Rohlf 2004a). The program TPSDig2 (Rohlf 2004b) was used to digitize morphological landmarks, which were homologous features that permit the general description of body form. 30 type I and II landmarks were recorded (Table 2).
The size of each configuration was estimated by using the “size centroid” (TC) that is calculated by taking the square root of the sum of the squares of the distance of each landmark from the centroid (Bookstein 1991, Bookstein et al. 1999). Once all images were digitized, the effects of translation, scale and rotation were eliminated from the set of configurations using an orthogonal analysis of generalized procrustes least squares (AGP) (Rohlf & Slice 1990), using the software program TPSSmall (Rohlf 2003); all configurations were scaled at TC=1 for this procedure.
The coordinate matrix generated by the TPS Dig2 program was analyzed with the MorphoJ program, which generated: an analysis of variance of canonical variables (AVC) using standardized data; this multivariate analysis maximizes the traditional morphological variation between groups; the changes in shape associated with each canonical variable (VC) that are drawn as deformation grids that describe the differences found between groups, and consists of the observations of the multivariate analysis compared with data obtained with traditional techniques (AVC), discriminating the variation between species; and evaluating the similarity among populations based on the Mahalanobis distance nearness.
Comparative material examined: See (Román-Valencia 2001, Román-Valencia et al. 2006, 2007, 2009a, 2009b, Román-Valencia & Ruiz-C. 2007, Román-Valencia & Arcila-Mesa 2008, 2009, 2010) for additional lists of comparative material examined of Hemibrycon species that we compared with this new species. Data were also used from Bertaco et al. (2007).
Results
Hemibrycon fasciatus n. sp.
(Table1-5, Figs. 2-3, 6-13)
Holotype: IUQ 3191, 81.0mm SL, Colombia, Antioquia, Municipio de San Vicente, Vereda Corrientes, middle sector of the Magdalena River Basin, La Magdalena Creek, tributary of the Nare River, 6°18’42.4” N-75°15’28.7’’ W, 1 882m.a.s.l., 27 Mar 2010, N. Mancera (Fig. 2).
Paratypes: All from the state (Departamento) of Antioquia, middle Magdalena River Basin and collected by N. Mancera: IUQ 3065 (4), 32.4-71.5mm SL, Quebrada Santa Gertrudis afluente río Nare, municipio de Concepción 6º19’21.0” N-75º09’38.6” W, 1 820m.a.s.l., 31 Jan 2010. IUQ 3156 (1), 52.7mm SL, Quebrada Santa Gertrudis, afluente río Nare, límites municipios Concepción y Alejandría, después del embalse Peñol-Guatapé 6º19’32.3” N-75º09’50.4” W, 1 820m.a.s.l., 22 Jun 2011. IUQ 3192 (2, C&S), 52.0-58.8mm SL, Quebrada Santa Gertrudis afluente río Nare, municipio de Concepción 6º19’21.0” N-75º09’38.6” W, 1 820m.a.s.l., 31 Jan 2010. IUQ 3160 (1), 44.3mm SL, Quebrada Santa Gertrudis, afluente río Nare, municipio de Concepción, 6º19’21.0” N-75º09’38.6” W, 1 820m.a.s.l., 22 Jun 2011. IUQ 3168 (1), 72.2mm SL, Quebrada La Magdalena, vereda Corrientes, municipio San Vicente, afluente río Nare 6°19’52.5” N-75°17’04.8” W, 2 140m.a.s.l., 24 Jun 2011. IUQ 3167 (1), 59.4mm SL, Quebrada La Magdalena afluente río Nare, municipio San Vicente, antes del embalse Peñol-Guatapé 6°19’52.5’’ N-75°17’4.8” W, 2 140m.a.s.l., 17Nov 2011. IUQ 3173 (1), 73.1mm SL, Quebrada La Magdalena, afluente río Nare, municipio San Vicente, 6°18’42.4” N-75°15’28.7’’ W, 1882 25 Sep 2011. IUQ 3171 (1), 55.2mm SL, Quebrada La Magdalena, vereda Corrientes, municipio San Vicente, afluente río Nare 6°19’52.5’’ N-75°17’4.8” W-2 140m.a.s.l., 24 Jun 2011. IUQ 3164 (2), 75.1-91.1mm SL, Quebrada La Magdalena, afluente río Nare, vereda Corrientes, municipio San Vicente, afluente río Nare 6°18’42.4’’ N-75°15’28.7” W, 1 882m.a.s.l., 24 Jun 2011. IUQ 3166 (2), 74.9-76.7mm SL, Quebrada La Magdalena, vereda Corrientes, municipio San Vicente, afluente río Nare 6°19’52.5’ ’N-75°17’04.8” W, 2 140m.a.s.l., 17 Nov 2011. IUQ 3070 (24), 45.0-82.8mm SL, Quebrada La Magdalena, vereda Corrientes, 6°18’42.4’’ N-75°15’28.7” W, 1 882m.a.s.l. 27 Mar 2010. CSJ 14 (2), 50.2-54.6mm SL, Quebrada La Magdalena, vereda Corrientes, 6°18’42.4’’ N-75°15’28.7” W, 1 882m.a.s.l. 27 Mar 2010. AUM 56756 (1), 62.4mm SL, Quebrada La Magdalena, vereda Corrientes, 6°18’42.4’’ N-75°15’28.7” W, 1 882m.a.s.l. 27 Mar 2010. IUQ 3078 (8), 23.6-65.8mm SL, Quebrada Santa Gertrudis, afluente río Nare, municipio de Concepción 6º19’21.0” N-75º09’38.6” W, 1 820m.a.s.l., 26 May 2008. IUQ 3158 (1), 58.1mm SL, Quebrada Santa Gertrudis, afluente río Nare, municipio de Concepción 6º19’21.0” N-75º09’38.6” W, 1 820m.a.s.l., 22 Jun 2011. IUQ 3193 (1, C&S), 61.3mm SL, Quebrada La Magdalena, vereda Corrientes, 6°18’42.4’’ N-75°15’28.7” W, 1 882m.a.s.l., 27 Mar 2010.
Diagnosis: Hemibrycon fasciatus n. sp. differs from H. mikrostiktos, H. boquiae, H. brevispini, H. microformaa, H. metae, H. colombianus, H. rafaelense and H. palomae by the presence of a circular humeral spot that does not pass ventrally below the lateral-line canal, and has a only a diffuse, inconspicuous vertical extension (vs. presence of a vertically elongate humeral spot that reaches 1 or 2 scales below the lateral line canal); and by having short scales with a rounded posterior margin at the base of the caudal-fin (vs. scales at base of caudal fin elongate and with posterior margin lobed, Fig. 3). It differs from the other species of Hemibrycon (H. beni, H. helleri, H. huambonicus, H. inambari, H. jelskii, H. polyodon, H. surinamensis, H. taeniurus, H. divisorensis, H. quindos, H. santamartae, H. raqueliae, H. virolinica, H. yacopiae and H. jabonero), in having melanophores present on the posterior margins of the scales all along the sides of body (vs. melanophores absent from margins of scales along entire length of sides of body; except for H. virolinica); by having a dark lateral stripe, formed by deep pigment that is continuous with the caudal peduncle spot (vs. lateral stripe silvery); by a wide, concave pelvic bone (vs. narrow and straight); by having the middle part of the dorsal margin of the orbito-sphenoid bone concave, and not in contact with the frontal (vs. in contact with frontal); ventral tip of extrascapular bifurcate (vs. not bifurcate); first postcleithrum not contacting cleithrum (vs. touching). The portion of maxilla with teeth does not reach the dorsal border of the dentary (vs. portion of maxilla with teeth reaching beyond dorsal border of dentary); all maxillary teeth tricuspid (vs. some unicuspid teeth present on maxilla); it differs from H. virolinica by having the caudal peduncle spot continuous with the dark lateral stripe (vs. melanophores of caudal peduncle spot not conspicuous and restricted to just caudal peduncle) and by having the humeral spot extending on to the posterior part of the opercle (vs. area anterior to humeral spot depigmented).
Description: Body slender and elongate (mean maximum body depth about 29.6% SL). Area above orbits convex, concave between posterior margin of orbit and supraoccipital spine. Dorsal profile of head and body oblique from supraoccipital to dorsal-fin origin and from last dorsal-fin ray to base of caudal fin. Ventral profile of body convex from snout to base of pelvic fin; straight from pelvic-fin origin to anal fin. Caudal peduncle laterally compressed. Head and snout short (21.7% SL and 26.3% HL respectively), jaws equal, mouth terminal, lips soft and flexible, and not covering outer row of premaxilla teeth; ventral border of upper jaw straight; posterior edge of maxilla reaching anterior edge of orbit.
Premaxilla with two rows of teeth. Two-four teeth of outer row tricuspid with central cusp larger. Inner premaxillary row with four pentacuspid teeth that diminish gradually in size. Maxilla long, posterior margin straight, with 6-11 uni- or tricuspid teeth, central cusp slightly longer. Dentary with four large tricuspid teeth with central cusp largest, followed by four to nine smaller, uni- to tricuspid teeth. Total number of vertebra 38-40. Six infraorbitals present, the first thin and narrow, extending between the dorsal edge of maxilla and lateral ethmoid, with sensorial canal. Second infraorbital short and wide, not covering dorsal part of angulo-articular. Anterior part of second infraorbital overlaying anterior part of first infraorbital; its posterior margin extends below third infraorbital. Third infraorbital widest and longest, its ventral border in contact with sensorial canal of preopercle. Fourth, fifth and sixth infraorbitals short and wide, covering posterior margin of hyomandibular. Supraorbital absent. Eight to nine supraneurals present between head and anterior part of dorsal fin, without cartilage on upper and lower edges, and with medial sensorial canal.
Secondary sexual dimorphism: Between 13-18 spines present on seventh branched pelvic-fin ray, located on both branches of ray, but predominant on internal branch; the largest spine at distal tip of ray, then gradually reduced in size. Spines absent from simple pelvic-fin rays. Males have a row of very short hooks on first four or five simple anal-fin rays, and long hooks on first seven or eight branched anal-fin rays, each ray has from 6-14 hooks, that extend along both sides of posterior margins of rays.
Pigmentation in alcohol: Body dark brownish-yellow, cromatophores more densely concentrated on dorsum, most intense on head. Midlateral body with dark stripe from posterior margin of humeral spot to caudal peduncleand prolonged onto middle caudal-fin rays. Humeral spot circular, located just behind posterior margin of opercle, not reaching the second series of scales below the lateral-line canal. Ventral part of body light yellow. Posterior margins of scales dark on dorsal region of body. Dorsal fin with cromatophores concentrated mostly on interradial membranes and distal margins of anterior rays. Adipose fin dark. Cromatophores on middle caudal-fin rays, near caudal-fin base but hyaline distally. Pectoral and pelvic fins hyaline; anal and caudal- fin lobes dusky.
Distribution: This species is so far known only from the Nare River drainage, Magdalena River basin, Colombia.
Comments: H. fasciatus occurs with H. virolinica and may be phylogenetically related to it, however, it can be distinguished from it by the presence of a dark lateral stripe that is absent in H. virolinica and by the presence of pigment on the adipose fin (vs. absent).
Etymology: fasciatus is from the Latin fascia, alluding to the dark lateral stripe or band that distinguishes this species. To be treated as a noun in apposition.
Hemibrycon cardalensis n. sp.
(Tables 1-5, Fig. 4, 6-9, 11-12)
Holotype: IUQ 3190, male, 78.2mm SL, Colombia, Antioquia, border between Municipios San Rafael and San Carlos, above Playas Reservoir, El Cardal Creek, tributary of Guatapé River, middle sector of the Magdalena River Drainage,6º16’56.4” N-74º55’37.7” W, 898m.a.s.l., 28 Mar 2010, N. Mancera (Fig. 4).
Paratypes: All from the state (Departamento) of Antioquia, middle sector of the Magdalena River Basin, collected by N. Mancera: IUQ 3049 (4), 52.5-63.8mm SL, límites de los Municipios San Rafael y San Carlos, después del embalse de Playas, Quebrada El Cardal, afluente río Guatapé, 06°16’56,4’’ N-74°55’37.7’’ W, 898m.a.s.l., 9 Mar 2008. IUQ 3046 (1), 69.1mm SL, Quebrada El Cardal, afluente río Guatapé, después del embalse Playas, 06°16’56.4’’ N-74°55’37.7’’ W 898m.a.s.l., 18 Nov 2007. IUQ 3048 (4), 53.7-79.9mm SL, Quebrada El Cardal, afluente río Guatapé, después del embalse Playas, 06°16’ 56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 9 Mar 2008. IUQ 3050(8), 53.5-84.7mm SL, Quebrada El Cardal, afluente río Guatapé, 06°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 28 Mar 2010, col. N. Mancera. IUQ3051 (9), 53.5-84.8mm SL, Quebrada El Cardal, 27 Jan 2010. CSJ 146 (2), 54.2-55.3mm SL, Quebrada El Cardal, 06°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 27 Jan 2010. AUM 56755 (1), 61.4mm SL, Quebrada El Cardal, 27 Jan 2010. IUQ3052 (14), 40.0-73.2mm SL, Quebrada El Cardal afluente río Guatapé, después del embalse Playas 06°16’56.4’’ N-74°55’37.7” W, 898m.a.s.l., 9 Mar 2008. IUQ 3054 (3), 30.8-53.9mm SL, Quebrada El Cardal, afluente río Guatapé, 6°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 18 Nov. 2007. IUQ 3055 (1), 68.1mm SL, Quebrada El Cardal afluente río Guatapé, después del embalse Playas, 06°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 14 Oct 2007. IUQ 3072(1), 53.4mm SL, Quebrada El Cardal, afluente río Guatapé, 06°16´56,4” N-74°55´37.7” W, 898m.a.s.l., 9 Mar 2008. IUQ 3073 (4), 49.5-63.1mm SL, Quebrada El Cardal, afluente río Guatapé, 6°16’56.4’’ N-74°55’37.7’ W, 898m.a.s.l., 28 Mar 2010. IUQ 3077 (2), 51.9-62.7mm SL, Quebrada El Cardal, afluente río Guatapé, 6°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 14 Oct 2007. IUQ 3161 (2), 49.4-57.5mm SL, Quebrada El Cardal, afluente río Guatapé, 24 May 2008. IUQ 3162 (1), 82.4mm SL, Quebrada El Cardal, 06°16’47.5’’ N-74°56’27.4’’ W, 976m.a.s.l. 21 Jun 2011. IUQ 3155 (1), 68.1mm SL, Quebrada El Cardal, afluente río Guatapé, 06°16’46.6’’ N-74°55’24.6’’ W, 898m.a.s.l., 21 Jun 2011. IUQ 3159 (1), 77.7mm SL, Quebrada El Cardal, afluente río Guatapé, 06°16’46.6” N-74°55’24.6’’ W, 898m.a.s.l., 22 Sep 2011. IUQ 3152 (2), 52.1-72.8mm SL, Quebrada El Cardal, afluente río Guatapé, 6°16’46.6’’ N-74°55’24.6’’ W, 898m.a.s.l., 20 Jun 2011. IUQ 3056 (2 C & S), 56.2-67.2mm SL, Quebrada El Cardal afluente río Guatapé, 9 Mar 2008. IUQ 3057 (2, C&S), 59.4-71.6mm SL, Quebrada El Cardal afluente río Guatapé, después del embalse Playas, 6°16’56.4’’ N-74°55’37.7’’ W, 898m.a.s.l., 24 May 2008.
Diagnosis: H. cardalensis n. sp. Differs from all congeners, including the sympatric species and H. fasciatus n. sp., described herein, in having the dark pigment of the peduncle spot restricted to just the ventral half of the caudal peduncle (vs. pigment distributed symmetrically above and below central axis of caudal peduncle), in having melanophores outlining the anterior lateral-line scales (vs. anterior lateral-line scales not outlined with melanophores) and in having scales of posterior margin of caudal peduncle with multiple undulations (vs. scales at posterior margin of caudal peduncle with bilobed or rounded posterior margins, Fig. 3).
Description: Body slender and elongate (mean maximum body depth about 30.5% SL). Area above orbits straight from snout to tip of supraoccipital spine, predorsal area extending above surface of cranium. Dorsal profile straight from supraoccipital spine to dorsal-fin origin and convex from last dorsal-fin ray to base of caudal fin. Ventral profile of body convex from snout to base of pelvic fin; straight from origin of pelvic fin to anal fin. Caudal peduncle laterally compressed. Head and snout short (22.9% SL and 26.2% HL respectively), jaws equal, mouth terminal, lips soft and flexible, and not covering outer row of premaxilla teeth; ventral border of upper jaw straight; posterior edge of maxilla reaching anterior edge of orbit.
Premaxilla with teeth in two rows. Second to fourth teeth of outer row tricuspid with central cusp larger. Inner row with four pentacuspid teeth that diminish gradually in size. Maxilla long, its posterior margin straight, with 2-3 pentacuspid teeth near dorsal tip followed by 3-4 tricuspid teeth with slightly longer central cusps, followed by 1-3 unicuspid teeth. Dentary with four large tricuspid teeth with central cusp largest, followed by four to seven smaller teeth, uni-to tricuspid teeth. Total number of vertebra 39-40. Six infraorbitals present, the first thin and narrow, extending between dorsal edge of maxilla and lateral ethmoid, with sensorial canal. Second infraorbital short and wide, covering dorsal part of angulo-articular, anterior part of second infraorbital overlaying anterior part of first infraorbital, its posterior margin extends below third infraorbital. Third infraorbital widest and longest, its ventral border in contact with the sensorial canal of preopercle. Fourth, fifth and sixth infraorbitals short and wide, covering posterior margin of hyomandibular. Supraorbital absent. Seven to eight supraneurals present between head and anterior part of dorsal fin, sometimes with cartilage along upper and lower edges, and with medial sensorial canal.
Secondary sexual dimorphism: Males have row of 12-14 large hooks on fourth to fifth simple anal-fin rays, and on first 12 to 13 branched anal-fin rays, each ray with 6-14 hooks, located posterior to each side of central axis of ray shaft. There are also 13-18 hooks on all pelvic-fin rays that diminish in size from distal tip of each ray. Long hooks are also present on first five or six anterior dorsal-fin rays, with 8 to 14 hooks on each ray found all along ray’s margin to distal tip.
Life colors: Adults are counter-shaded with silvery lateral stripe highlighted by iridescent yellowish-green, more conspicuous along dorsal margin of lateral stripe, extending anteriorly on to dorsal margin of eye and posteriorly along dorsal and ventral margins of lateral stripe on caudal peduncle. Dorsal margin of opercle anterior to lateral stripeintense yellow. Infraorbital along posterior margin of orbit green, this color extending along dorsal half of opercle. Head beneath orbit violet, circumscribing third infraorbital. Scales on sides of body without melanophores, giving it a whitish or silvery appearance. Dorsal region bluish-green. Humeral spot wide, dark, and conspicuous beneath silvery lateral stripe. Posterior part of caudal peduncle with dark midlateral stripe that extends onto middle caudal-fin rays. Lower caudal fin lobe and tips of both lobes yellow. Pectoral and pelvic fins hyaline, anal and dorsal fins with a blackish bar crossing middle parts of rays, distal tips of dorsal and caudal fins dark.
Pigmentation in alcohol: Body dark brownish-yellow, cromatophores more densely concentrated on dorsum, most intense on head. Midlateral body with silvery stripe from posterior margin of humeral spot to caudal peduncle. Humeral spot vertically elongate, located justbehind posterior margin of opercle, extending from third scale of lateral line series. Ventral part of body light yellow. Posterior margin of scales on dorsal region of body brownishyellow. Dorsal fin with cromatophores concentrated mostly on interradial membranes and distal margins of anterior rays. Adipose fin hyaline. Caudal fin with dark cromatophores on middle rays. Anal, pectoral and pelvic fins hyaline. Anal as well as caudal-fin lobes with concentrations of dark cromatophores on both the rays and interradial membranes.
Distribution: This species is so far known only from El Cardal creek, a tributary of theGuatapé River; middle Magdalena River Basin, Colombia.
Etymology: Named cardalensis for El Cardal creek, where the type series was collected. To be treated as a noun in apposition.
Hemibrycon antioquiae n. sp.
(Tables 1-5, Fig. 5-12)
Holotype: IUQ 3189, 75.3mm SL, state of Antioquia, San Rafael County, below Playas Reservoir, Peñoles Creek, a tributary of the Guatapé River, Middle Magdalena River Basin, 06°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l.,19 Jun 2011, N. Mancera (Fig. 5).
Paratypes: All from the state of Antioquia, middle Magdalena River Basin, and collected by N. Mancera: IUQ 3069 (2, C&S), 57.7-58.2mm SL, municipio Alejandría, Quebrada San José, antes de la desembocadura al embalse San Lorenzo, cuenca río Nare, 6º21’59.1” N-75º03’26.7” W 1 261m.a.s.l., 10 Oct 2007. IUQ 3071 (4), 48.3-77.1mm SL, Río Guatapé, 06°16’52’’ N-75°06’0.6’ W, 1 209m.a.s.l., 15 Nov 2007. IUQ 3060 (2), 73.0-78.4mm SL, municipio de San Rafael, antes del embalse de playas, Quebrada Mazorcos, afluente del río Guatapé 06°16’69.6’’ N-75°06’0.27’’ W, 1 206m.a.s.l., 5 Mar 2008. IUQ 3195 (1, C&S), 68.8mm SL, río Guatapé, 06°16’52’’ N-75°06’0.6’’ W, 1 209m.a.s.l., 15 Nov 2007. IUQ 3068 (1), 54.1mm SL, municipio de Alejandría, Quebrada San Lorenzo antes de la desembocadura al embalse San Lorenzo, cuenca río Nare 6º22’19” N-75º03’25.5” W, 1 250m.a.s.l., 29 Jan 2010. IUQ 3194 (1, C&S), 63.5mm SL, municipio de Alejandría, Quebrada San Lorenzo antes de la desembocadura al embalse San Lorenzo, cuenca río Nare 6º22’19” N-75º03’25.5” W, 1 250m.a.s.l., 29 Jan 2010. IUQ 3058 (6), 53.6-76.7mm SL, municipio de San Rafael, antes del embalse de playas, Quebrada Mazorcos, afluente del río Guatapé, 25 May 2008. IUQ 3063 (1), 63.3mm SL, municipio de San Rafael, antes del embalse de playas, Quebrada Peñoles afluente Río Guatapé, 6°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l., 15 Nov 2007. IUQ 3064 (1, C&S), 64.4mm SL, Quebrada Peñoles, afluente Río Guatapé 6°16’26.7’’ N-75°05’22.9” W, 1 201m.a.s.l., 15 Nov 2007. IUQ 3059 (2), 68.4-81.0mm SL, Quebrada Peñoles, afluente Río Guatapé, 6°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l. 28 Mar 2010. IUQ 3047(1), 56.6mm SL, municipio San Vicente, antes del embalse Peñol-Guatapé 06°14’59.1’’ N-75°19’29.6’’ W, 2 094m.a.s.l., Quebrada La Compañía afluente río Nare, 27 May 2008. IUQ 3074 (1), 59.7mm SL, limites municipios San Rafael-San Carlos, después del embalse de playas, Quebrada el Cardal afluente río Guatapé, 6º16’46.6” N-74º55’24.6” W, 898m.a.s.l., 24 May 2008. IUQ 3067 (2), 44.7-46.6mm SL, municipio de Alejandría, Quebrada San Lorenzo antes de la desembocadura al embalse San Lorenzo, cuenca río Nare 6º22’19” N-75º03’25.5” W, 1 250m.a.s.l., 29 Jan 2010. IUQ 3061(2), 67.7-73.8mm SL, Quebrada Peñoles afluente río Guatapé, 6°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l., 25 May 2008. IUQ 3076 (1), 65.8mm SL, Quebrada El Cardal, afluente río Guatapé, 6º16’46.6” N-74º55’24.6” W, 898m.a.s.l., 24 May 2008. IUQ 3053(10), 40.7-67.2mm SL, Quebrada El Cardal afluente Río Guatapé, 6º16’46.6” N-74º55’24.6” W, 898m.a.s.l., 24 May 2008. IUQ 3066 (8), 45.1-62.3mm SL, Quebrada San José, antes de la desembocadura al embalse San Lorenzo, cuenca río Nare, 6º21’59.1” N-75º03’26.7” W, 1 261m.a.s.l., 10 Oct 2007. IUQ 3075(1), 83.9mm SL, Quebrada Peñoles afluente río Guatapé, 06°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l., 15 Nov 2007. IUQ 3062 (5), 51.8-59.8mm SL, Quebrada Peñoles, afluente Río Guatapé 6°16’26.7’’ N-75°05’22.9’’ W, 1 201m.a.s.l., 15 Nov 2007. IUQ 3163 (8), 54.8-67.3mm SL, Quebrada Peñoles afluente río Guatapé, 6°16’04.4’’ N-75°05’12.7’’ W, 1 246m.a.s.l., 19 Jun 2011. CSJ 145 (2), 56.2-57.3mm SL, Quebrada Peñoles afluente río Guatapé, 6°16’04.4’’ N-75°05’12.7’’ W, 1 246m.a.s.l., 19 Jun 2011. AUM 56754 (1), 55.2mm SL, Quebrada Peñoles afluente río Guatapé, 6°16’04.4’’ N-75°05’12.7’’ W, 1 246m.a.s.l., 19 Jun 2011. IUQ 3158 (1), 68.9mm SL, Quebrada El Cardal afluente río Guatapé, 6º16’47.5” N-74º56’27.4” W, 976m.a.s.l., 14 Nov 2011. IUQ 3150 (1), 78.3mm SL, Quebrada El Cardal, afluente Río Guatapé, 6º16’37.5” N-74º55’47.9” W, 950m.a.s.l., 14 Nov 2011. IUQ 3153 (2), 61.3-62.1mm SL, Quebrada Peñoles afluente río Guatapé, 6°15’49.6’’ N-75°05’06.4’’ W, 1 265m.a.s.l., 20 Jun 2011. IUQ 3157 (1), 70.8mm SL, Quebrada Peñoles afluente río Guatapé, 6°16’04.4’’ N-75°05’12.7’’ W, 1 246m.a.s.l. 19 Jun 2011. IUQ 3154 (2), 81.2-82.4mm SL, Quebrada El Cardal afluente río Guatapé, 6º16’46.6” N-74º55’24.6” W, 898m.a.s.l., 22 Sept. 2011. IUQ 3170 (8), 42.1-79.2mm SL, Quebrada El Cardal afluente Río Guatapé, 6º16’46.6” N-74º55’24.6” W, 898m.a.s.l., 27 Jan 2010. IUQ 3169 (1), 43.9 mm SL, Quebrada La Magdalena, afluente río Nare, municipio de San Vicente, vereda Corrientes, 6°18’42.4” N-75°15’28.7’’ W, 1 882m.a.s.l., 17 Nov 2011. IUQ 3165 (2), 74.3-75.5mm SL, Quebrada Peñoles, afluente Río Guatapé, 6°16’26.7’’ N-75°05’22.9” W, 1 201m.a.s.l., 5 Mar 2008.
Diagnosis: Hemibrycon antioquiae n. sp. differs from the remaining species of Hemibrycon that also have circular humeral spots, such as H. mikrostiktos, by having a projection of diffuse melanophores extending from the dorsal margin of the humeral spot that pass over the lateral stripe (vs. dorsal border of humeral spot without projections of diffuse melanophores). It differs from H. metae, H. colombianus, H. boquiae, H. rafaelense and H. cairoense in having a protruding predorsal area, with respect to the dorsal surface of the cranium (vs. predorsal area with same degree of inclination as the surface of cranium), by the presence of a dark lateral stripe and in having the peduncular spot extending over the lateral stripe (vs. lateral stripe silvery, without melanophores, and peduncular spot not conspicuous). It differs from H. fasciatus n. sp. in having the posterior margin of infraorbital 3, 4 and 5 in contact with the preopercle (vs. infraorbitals 3, 4 and 5 not in contact with preopercle). And differs from all congeners in having the posterior margin of the cleithrum rectangular (vs. in form of arc, or sigmoid) and is distinguished from H. cardalensis n. sp. by vertically elongate humeral spot that passes through the lateral-line canal ventrally (vs. humeral spot does not pass the lateral-line canal ventrally).
Description: Body slender and elongate (mean maximum body depth about 29.1% SL). Dorsal profile of cranium straight less inclined than adjacent predorsal region. Profile oblique from last dorsal-fin ray to base of caudal fin. Ventral profile of body convex from snout to base of anal fin. Caudal peduncle laterally compressed. Head and snout short, mandibles equal, mouth terminal, lips soft and flexible, and not covering outer row of premaxilla teeth; ventral border of upper jaw straight; posterior edge of maxilla reaching anterior edge of orbit.
Premaxillary teeth in two rows. Second to fourth teeth of outer row tricuspid with central cusp larger. Internal row with four pentacuspid teeth that diminish gradually in size. Maxilla long, posterior margin straight, 8-11 tricuspid teeth with central cusp slightly longer. Dentary with four large tricuspid teeth with central cusp largest, followed by four to nine smaller teeth with one to three cusps. Total number of vertebra 36-43. Six infraorbitals present, the first thin and narrow, extending between dorsal edge of maxilla and lateral ethmoid, with sensorial canal. Second infraorbital short and wide, covering dorsal part of the angulo-articular, anterior part of second infraorbital overlaying anterior part of first infraorbital with a foramen that extends towards dorsal margin of first infraorbital; its posterior margin extends below third infraorbital. Third infraorbital widest and longest, its ventral border in contact with sensorial canal of preopercle. Fourth, fifth and sixth infraorbitals short and narrow, covering posterior margin of hyomandibular. Supraorbital absent. Seven to eight supraneurals present between head and anterior part of dorsal fin, without cartilage on upper and lower edges, and with medial sensorial canal.
Secondary sexual dimorphism: Males have a row of very short spines on the first to fourth or fifth branched rays, each ray with from 7-13 spines, located on central shaftand directed posterior to it, on all branches of ray. There are also from 9-13 small spines on all branched rays of pelvic fin, located on both branches of rays, extending along entire length of ray.
Pigmentation pattern in alcohol: Body dark, cromatophores more densely concentrated on dorsum, most intense on head. Humeral spot located just behind posterior margin of opercle extending from first scale of lateral line series, just above spot and posterior to thin unpigmented there is a second diffuse projection of melanophores. Ventral and midlateral anterior part of body light yellow. Posterior margins of scales on dorsal region of body dark. Dorsal fin with cromatophores concentrated mostly on interradial membranes and distal margins of anterior rays. Adipose fin hyaline. Caudal fin with dark cromatophores on middle rays. Anal, pectoral and pelvic fins hyaline. Anal as well as caudal-fin lobes with dark cromatophores on rays and interradial membranes but with hyaline regions in anterior part of both fins.
Distribution: This species is so far knownonly from the Nare River drainage, River Magdalena basin, Colombia.
Etymology: Hemibrycon antioquiae n. sp. is named for Antioquia state, Colombia, where the type series was collected. It is to be treated as a noun in apposition.
Ecological notes: The pH of the water ranged from 6.9 (Quebrada Santa Gertrudis, locality of H. fasciatus) and 8.7 (Quebrada La Magdalena, Station 1, H. antioquiae); dissolved oxygen was from 4.2mg/L, and 55% saturation (Quebrada San Juan, H. antioquiae) and 9.1mg/L, with 113.3% saturation (Quebrada Peñoles, station 1, H. antioquiae); conductivity ranged from 16.4μs/cm (Quebrada Peñoles station 2, H. antioquiae) and 52.7μs/ cm (Quebrada El Cardal station 3, H. cardalensis). Surface water temperature ranged from 18o C (in Quebrada La Magdalena, station 1, habitat of H. antioquiae) and 22oC (in Quebrada El Cardal, station 2, habitat of H. antioquiae and H. cardalensis).
Morphological analysis: H. antioquiae is similar to H. virolinica, but can be distinguished by the presence of a dark lateral stripe on the body (vs. dark stripe absent in H. virolinica) and the presence of pigment on the adipose fin (vs. pigment absent).The principal component analysis (PCA) on traditional morphological data of the new and described species of Hemibrycon from the region revealed differences of the three new species described here from H. colombianus, H. palomae and H. yacopiae; in the first principal component axis postorbital length of the head and pelvic-fin length were the most important variables. In the second component caudal peduncle length and pectoral-fin length were most significant. Distance between dorsal fin and adipose fin and upper jaw length were the most important for component three. The first component explained 55.72% of total variability, summed with the second 92.87 %, and between the first and third 58.48% (Fig. 6; Tables 3-4).
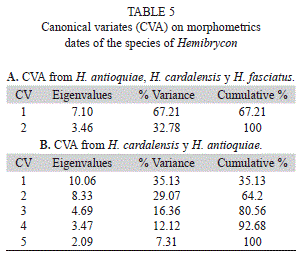
The morfogeometric analysis using Canonical Variable Analysis (CVA) discriminated the included species of Hemibrycon (Fig. 7-8, Tables 5); two canonical variables (VC) expressed a generalized pattern of contraction or extension of the cranium. Among the most notable transformations observed we found: snout length; transverse modification of the middle part of the body (the area enclosed between the tips of the dorsal fin and the pelvic-fin origins and the pelvic-fin origins and the anal fin insertion); contraction of the prepelvic area and displacement along a vertical axis of the melanophores in the humeral region. Canonical variable 1 explained 67.21% of the previously described morphological variation (Fig. 7), and is related to the vertical displacement of the melanophores in the humeral region (landmarks 21 and 22), contraction of the snout (landmarks 1, 2 and 3) and depression of the predorsal region (4, 5, 6 and 7, Fig. 8). Canonical variable 2 explained 32.78% (Fig. 7, Table 5A), and is associated, among several characteristics, with the prolongation of the snout (landmarks 1, 2 and 3), dorsal projection of the orbit (27 and 28) and the posterior ventral placement of the humeral spot melanophores.
The divergence observed between H. fasciatus and H. antioquiae, both of which are widely distributed throughout the study area, was explained completely by just one canonical variable (Fig. 9A), which described the following transformations: contraction between the dorsal and adipose fins (landmarks 9 and 10), increased caudal peduncle depth (11-15) and expansion of the predorsal area (landmarks 6-8, Fig. 9B). H. cardalensis is endemic to El Cardal Creek, and is sympatric with H. antioquiae, which have a wider distribution throughout the region. The morphological divergence between H. cardalensis and H. antioquiae, was explained by five canonical variables (Fig. 10A), of which the first three explained 80.5% of total variation (Table 5B), and are defined by contraction of the orbit (landmarks 3-4, and 27-28), the preanal region (17-18) and predorsal region (5-7) VC1 (Fig. 10B), ventral displacement of the melanophores of the humeral spot (landmarks 21-22) and prolongation of the caudal peduncle (landmarks 10-11, 15-16) VC2 (Fig. 10C); and also by the contraction of the dorsal-fin length (landmarks 8-9) and depression of the dorsal surface of the caudal peduncle (10 and 11) VC3 (Fig. 10D). The divergence of H. cardalensis from H. fasciatus in a morphological sense is a generalized contraction of the type IA landmark, as is explained completely with one canonical variable (Fig. 11A-B).
The discriminant function analysis present Mahalanobis Distance (H. antioquiae - H. cardalensis, (5.3); H. antioquiae - H. fasciatus (5.2); H. fasciatus - H. cardalensis (7,4), indicating that H. fasciatus is better diagnosed, perhaps indicating, based on an analysis of its populations, that it was naturally present in the Nare River drainage (Fig. 12).
Discussion
H. antioquiae shows, among its populations, a more generalized form with regards to other Hemibrycon species described herein; this indicates a greater association with drainages of the Guatapé River, from where it could have dispersed to reach its current distribution. Its presence in the Guatapé River drainages, are the result of human actions related to the construction of San Lorenzo, Playas and Punchiná dams, that has allowed the current contact between these taxa, that were naturally isolated previously. The distribution of H. fasciatus was observed in the Santa Gertrudis Creek drainage at an altitude of 1 820m.a.s.l., and La Magdalena Creek at 2 140m.a.s.l, both streams are components of the Nare River drainage. The distribution of H. cardalensis was observed in El Cardal Creek, of the Guatapé River drainage at 898m.a.s.l., and H. antioquiae was widely distributed in La Magdalena Creek around 2 014m.a.s.l., San Lorenzo and San José Creeks between 1 250 and 1 261m.a.s.l.in the Nare River drainage, in Peñoles, Mazorcos and El Cardal Creeks in the Guatapé River drainage. For El Cardal Creek, both H. cardalensis and H. antioquiae occurred. We inferred that H. antioquiae (the species with the wider distribution) may have occurred only in the Nare River drainage before the construction of the dams, and that it arrived in the Guatapé River drainage via connections formed after dams constructions in Eastern Antioquia, since water is diverted from San Lorenzo dam (Nare drainage) to the Playas reservoir, and from there, to Punchiná reservoir (Guatapé River drainage), and thus possibly allowing the movement of H. antioquiae into the Guatapé river drainage, and Cardal Creek, where H. cardalensis is found. In this study we found H. fasciatus at an altitude of 2 140m.a.s.l. in La Magdalena Creek; this represents the first record of an Hemibrycon species above 1 819m.a.s.l., the previous altitudinal record reported for H. boquiae (Román- Valencia 2001, Román-Valencia et al. 2008).
One of the species we compared to the new species, H. pautensis, was proposed as a junior synonym of H. polyodon by Bertaco & Malabarba (2010), who noted that H. polyodon was not included among comparative material in the description of H. pautensis by Román-Valencia et al. (2006); nevertheless, Bertaco & Malabarba (2010) did not examine the type material of H. pautensis. Upon comparison of both contributions (Román-Valencia et al. 2006, Bertaco & Malabarba 2010) we noted the following differences between H. pautensis and H. polyodon: H. pautensis has a longer caudal peduncle (8.07-11.70 vs. 14.4-16.6% SL) and longer upper jaw (23.38-30.80 vs. 43.7-45.6 % SL); and a smaller orbital diameter (29.5-34.1 vs. 39.51-44.06% SL). Furthermore, H. pautensis and H. orcesi (=B. orcesi) have five to seven hypurals, whereas all other species of Hemibrycon examined, invariably have only five, presenting no variation. In light of these differences we considered H. pautensis to be a valid species and remove it from the synonymy of H. polyodon. Overall, the process of the groups biodiversity recognition and its systematics continues, with some new taxon descriptions mainly from Rio Cauca, and yet more information is being generated for the undocumented Magdalena-Cauca system. Key to the species of Hemibrycon from the Magdalena River Basin
Acknowledgments
We would like to thank ISAGEN E.S.P., Corporación Autónoma Regional de los Ríos Negro y Nare (CORNARE) and the Universidad Nacional de Colombia, Medellin, for financial assistance to NM-R., for project 20101009235: “A study of the biology, ecology and genetic diversity of the characid fish, Brycon henni, in the Nare and Guatapé River drainages, Antioquia, Colombia”, during which the specimens of Hemibrycon described in this study were collected. We also thank the Universidad del Quindío, Vicerrectoría de Investigaciones for financing investigations of C.R.-V. and R.I. Ruiz-C. on Hemibrycon of Colombia, Ecuador and Venezuela (grants 212 and 304).
References
Bertaco, V.A., L.R. Malabarba, M. Hidalgo & H. Ortega. 2007. A new species of Hemibrycon (Teleostei: Characiformes: Characidae) from the río Ucayali drainage, Sierra del Divisor, Perú. Neotrop. Ichthyol. 5: 251-257. [ Links ]
Bertaco, V.A. & L.R. Malabarba. 2010. A review of the Cis-Andean species of Hemibrycon Günther (Teleostei: Characiformes: Characidae: Stevardiinae), with description of two new species. Neotrop. Ichthyol. 8: 737-770. [ Links ]
Bookstein, F.L. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge University, Cambridge, US. [ Links ]
Bookstein, F.L., K. Schafer, H. Prossinger, H. Seidler, M. Fieder, C. Stringer, G. Weber, W.J.L Arsuaga, D. Slice, F.J Rohlf., W. Recheis, A.J. Mariam & L.F. Marcus .1999. Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat. Record 257: 217-224. [ Links ]
García-Alzate, C.A., R.I. Ruiz-C., C. Román-Valencia, M. González & D. Lopera. 2011. Morfología de las especies de Hyphessobrycon (Characiformes: Characidae), grupo heterorhabdus, en Colombia. Rev. Biol. Trop. 59: 709-725. [ Links ]
Hammer, Ø., D.A. Harper & P.D. Ryan. 2008. PASTPalaeontological Statistics, ver. 1.81: 1-88. [ Links ]
Rohlf, F.J. & D.E. Slice.1990.Extensions of the procruster method for the optimal superimposition of landmarks. Syst. Zool. 39: 40-50. [ Links ]
Rohlf, F.J. 2003. TpsSmall, version 1.20. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Rohlf, F.J. 2004a.tpsUtil, file utility program. version 1.26. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Rohlf, F.J. 2004b. tpsDig, digitize landmarks and outlines, version 2.0. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Román-Valencia, C. 2001. Redescripción de Hemibrycon boquiae (Pisces: Characidae), especie endémica de la Quebrada Boquia, cuenca del río Quindío, Alto Cauca, Colombia. Dahlia (Rev. Asoc. Colomb. Ictiol.) 4: 27-32. 695. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & R. Barriga. 2006. Una nueva especie de pez del Género Hemibrycon (Teleostei: Characidae). Rev. Biol. Trop. 54: 209-217. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & R. Barriga. 2007. Redescripción de Hemibrycon orcesi Böhlke, 1959 y H. Polyodon (Günther, 1864) (Teleostei, Characidae), incluye clave para las especies de Hemibrycon en Ecuador. Anim. Biodivers. Conserv. 30: 179-188. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2008. A new species of Hemibrycon (Characiformes:Characidae) from the upper Cauca River, with keys to Colombian species. Anim. Biodivers. Conserv. 31: 67-75. 714. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & A. Giraldo. 2008. Dieta y reproducción de dos especies sintópicas Bryconamericus caucanus y Hemibrycon boquiae (Pisces: Characidae) en la Quebrada Boquía, Alto Cauca, Colombia. Rev. Mus. Arg. Cienc. Nat., n.s. 10: 55-62. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2009. Two new species of Hemibrycon (Characiformes: Characidae) from the Magdalena river, Colombia. Anim. Biodivers. Conserv. 32: 77-87. [ Links ]
Román-Valencia, C., J.A. Vanegas-Ríos & M.D. García. 2009a. Análisis comparado de las especies de Bryconamericus (Teleostei: Characidae) en la cuenca de los ríos Cauca-Magdalena y Ranchería, Colombia. Rev. Mex. Biod. 80: 465-482. [ Links ]
Romá-Valencia, C., D.K. Arcila-Mesa & H. Hurtado. 2009b. Variación morfológica de los peces Hemibrycon boquiae y Hemibrycon rafaelense (Characiformes: Characidae) from the Río Magdalena basin, Colombia. Rev. Biol. Trop. 57: 541-556. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2010. Five new species Hemibrycon. Rev. Biol. Trop. 58: 339-356. [ Links ]
Román-Valencia, C., CA. Garcia-Alzate, R.I. Ruiz-C. & D.C. Taphorn B. 2010. New species of Hemibrycon (Characiformes, Characidae) from the Roble River, Alto Cauca, Colombia, with a key of species known from the Magdalena-Cauca Basin. Vert. Zool. 60: 99-105. [ Links ]
Ruiz-C., R.I. & C. Román-Valencia. 2006. Osteología de Astyanax aurocaudatus Eigenmann, 1913 (Pisces: Characidae), con notas sobre la validez de Carlastyanax Géry, 1972. Anim. Biodivers. Conserv. 29: 49-64. [ Links ]
Ruiz-C., R.I. & R. Cipriani. 2006. Análisis morfogeométrico de Astyanax siapae. Dahlia (Rev. Asoc. Colomb. Ictiol.) 9: 63-75. [ Links ]
Ruiz-C., R.I., C. Román-Valencia, B.E. Herrera-N., I.E. Peláez & A. Ermakova-A. 2011. Variación morfológica de las especies de Astyanax, subgénero Zygogaster. Anim. Biodivers. Conserv. (Teleostei, Characidae) 34.1: 47-66. [ Links ]
Sabaj-Perez, N.H. (ed.). 2012. Standard symbolic codes for institutional resource collections in Herpetology & Ichthyology: an on line reference, version 2.0. American Society Ichthyologist and Herpetologist, Washington, D.C., USA (Downloaded: November 8, 2010, http://www.asih.org/). [ Links ]
Song, J. & L.R. Parenti. 1995. Clearing and staining whole fish specimens for simultaneous demonstration of bone, cartilage and nerves. Copeia 114-118. [ Links ]
Taylor, W.R. & G.C. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9: 107-119. [ Links ]
Vari, R.P. 1995. The Neotropical fish family Ctenoluciidae (Teleostei: Ostariophysi: Characiformes): supra and intrafamilial phylogenetic relationship, with a revisionary study. Smith. Contr. Zool. 564: 1-96. [ Links ]
Vari, R.P. & D.J. Siebert. 1990. A new unusually sexually dimorphic species of Bryconamericus (Pisces: Ostariophysi: Characidae) from the Peruvian Amazon. Proc. Biol. Soc. 103: 516-524. [ Links ]
Weitzman, S.H. 1962. The osteology of Bryconmeeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol. Bull. 8: 1-77. [ Links ]
Bertaco, V.A. & L.R. Malabarba. 2010. A review of the Cis-Andean species of Hemibrycon Günther (Teleostei: Characiformes: Characidae: Stevardiinae), with description of two new species. Neotrop. Ichthyol. 8: 737-770. [ Links ]
Bookstein, F.L. 1991. Morphometric tools for landmark data: geometry and biology. Cambridge University, Cambridge, US. [ Links ]
Bookstein, F.L., K. Schafer, H. Prossinger, H. Seidler, M. Fieder, C. Stringer, G. Weber, W.J.L Arsuaga, D. Slice, F.J Rohlf., W. Recheis, A.J. Mariam & L.F. Marcus .1999. Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat. Record 257: 217-224. [ Links ]
García-Alzate, C.A., R.I. Ruiz-C., C. Román-Valencia, M. González & D. Lopera. 2011. Morfología de las especies de Hyphessobrycon (Characiformes: Characidae), grupo heterorhabdus, en Colombia. Rev. Biol. Trop. 59: 709-725. [ Links ]
Hammer, Ø., D.A. Harper & P.D. Ryan. 2008. PASTPalaeontological Statistics, ver. 1.81: 1-88. [ Links ]
Rohlf, F.J. & D.E. Slice.1990.Extensions of the procruster method for the optimal superimposition of landmarks. Syst. Zool. 39: 40-50. [ Links ]
Rohlf, F.J. 2003. TpsSmall, version 1.20. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Rohlf, F.J. 2004a.tpsUtil, file utility program. version 1.26. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Rohlf, F.J. 2004b. tpsDig, digitize landmarks and outlines, version 2.0. Department of Ecology and Evolution, State University of New York at Stony Brook, New York, US. [ Links ]
Román-Valencia, C. 2001. Redescripción de Hemibrycon boquiae (Pisces: Characidae), especie endémica de la Quebrada Boquia, cuenca del río Quindío, Alto Cauca, Colombia. Dahlia (Rev. Asoc. Colomb. Ictiol.) 4: 27-32. 695. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & R. Barriga. 2006. Una nueva especie de pez del Género Hemibrycon (Teleostei: Characidae). Rev. Biol. Trop. 54: 209-217. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & R. Barriga. 2007. Redescripción de Hemibrycon orcesi Böhlke, 1959 y H. Polyodon (Günther, 1864) (Teleostei, Characidae), incluye clave para las especies de Hemibrycon en Ecuador. Anim. Biodivers. Conserv. 30: 179-188. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2008. A new species of Hemibrycon (Characiformes:Characidae) from the upper Cauca River, with keys to Colombian species. Anim. Biodivers. Conserv. 31: 67-75. 714. [ Links ]
Román-Valencia, C., R.I. Ruiz-C. & A. Giraldo. 2008. Dieta y reproducción de dos especies sintópicas Bryconamericus caucanus y Hemibrycon boquiae (Pisces: Characidae) en la Quebrada Boquía, Alto Cauca, Colombia. Rev. Mus. Arg. Cienc. Nat., n.s. 10: 55-62. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2009. Two new species of Hemibrycon (Characiformes: Characidae) from the Magdalena river, Colombia. Anim. Biodivers. Conserv. 32: 77-87. [ Links ]
Román-Valencia, C., J.A. Vanegas-Ríos & M.D. García. 2009a. Análisis comparado de las especies de Bryconamericus (Teleostei: Characidae) en la cuenca de los ríos Cauca-Magdalena y Ranchería, Colombia. Rev. Mex. Biod. 80: 465-482. [ Links ]
Romá-Valencia, C., D.K. Arcila-Mesa & H. Hurtado. 2009b. Variación morfológica de los peces Hemibrycon boquiae y Hemibrycon rafaelense (Characiformes: Characidae) from the Río Magdalena basin, Colombia. Rev. Biol. Trop. 57: 541-556. [ Links ]
Román-Valencia, C. & D.K. Arcila-Mesa. 2010. Five new species Hemibrycon. Rev. Biol. Trop. 58: 339-356. [ Links ]
Román-Valencia, C., CA. Garcia-Alzate, R.I. Ruiz-C. & D.C. Taphorn B. 2010. New species of Hemibrycon (Characiformes, Characidae) from the Roble River, Alto Cauca, Colombia, with a key of species known from the Magdalena-Cauca Basin. Vert. Zool. 60: 99-105. [ Links ]
Ruiz-C., R.I. & C. Román-Valencia. 2006. Osteología de Astyanax aurocaudatus Eigenmann, 1913 (Pisces: Characidae), con notas sobre la validez de Carlastyanax Géry, 1972. Anim. Biodivers. Conserv. 29: 49-64. [ Links ]
Ruiz-C., R.I. & R. Cipriani. 2006. Análisis morfogeométrico de Astyanax siapae. Dahlia (Rev. Asoc. Colomb. Ictiol.) 9: 63-75. [ Links ]
Ruiz-C., R.I., C. Román-Valencia, B.E. Herrera-N., I.E. Peláez & A. Ermakova-A. 2011. Variación morfológica de las especies de Astyanax, subgénero Zygogaster. Anim. Biodivers. Conserv. (Teleostei, Characidae) 34.1: 47-66. [ Links ]
Sabaj-Perez, N.H. (ed.). 2012. Standard symbolic codes for institutional resource collections in Herpetology & Ichthyology: an on line reference, version 2.0. American Society Ichthyologist and Herpetologist, Washington, D.C., USA (Downloaded: November 8, 2010, http://www.asih.org/). [ Links ]
Song, J. & L.R. Parenti. 1995. Clearing and staining whole fish specimens for simultaneous demonstration of bone, cartilage and nerves. Copeia 114-118. [ Links ]
Taylor, W.R. & G.C. Van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9: 107-119. [ Links ]
Vari, R.P. 1995. The Neotropical fish family Ctenoluciidae (Teleostei: Ostariophysi: Characiformes): supra and intrafamilial phylogenetic relationship, with a revisionary study. Smith. Contr. Zool. 564: 1-96. [ Links ]
Vari, R.P. & D.J. Siebert. 1990. A new unusually sexually dimorphic species of Bryconamericus (Pisces: Ostariophysi: Characidae) from the Peruvian Amazon. Proc. Biol. Soc. 103: 516-524. [ Links ]
Weitzman, S.H. 1962. The osteology of Bryconmeeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol. Bull. 8: 1-77. [ Links ]
*Correspondencia a:
César Román-Valencia. Laboratorio de Ictiología, A. A. 2639, Universidad del Quindío, Armenia, Quindío, Colombia; ceroman@uniquindio.edu
Raquel I. Ruiz-C.. Laboratorio de Ictiología, A. A. 2639, Universidad del Quindío, Armenia, Quindío, Colombia; zutana_1@yahoo.com
Donald C. Taphorn. 1822 North Charles Street, Belleville, Illinois, 62221, USA; taphorn@gmail.com
Néstor J. Mancera-Rodriguez. Universidad Nacional de Colombia, Departamento de Ciencias Forestales, Sede Medellín, Calle 59A No. 63-20, Bloque 20, oficina 21, Medellín, Colombia; njmancer@unal.edu.co
Carlos A. García-Alzate. Laboratorio de Ictiología, A. A. 2639, Universidad del Quindío, Armenia, Quindío, Colombia; Departamento de Biología, Universidad del Atlántico, Km 7 antigua vía a Puerto Colombia, Barranquilla, Atlántico Colombia; carlosgarciaa@mail.uniatlantico.edu.co
1. Laboratorio de Ictiología, A. A. 2639, Universidad del Quindío, Armenia, Quindío, Colombia; ceroman@uniquindio.edu.co, zutana_1@yahoo.com
2. Universidad Nacional de Colombia, Departamento de Ciencias Forestales, Sede Medellín, Calle 59A No. 63-20, Bloque 20, oficina 21, Medellín, Colombia; njmancer@unal.edu.co
3. 1822 North Charles Street, Belleville, Illinois, 62221, USA; taphorn@gmail.com
4. Departamento de Biología, Universidad del Atlántico, Km 7 antigua vía a Puerto Colombia, Barranquilla, Atlántico Colombia; carlosgarciaa@mail.uniatlantico.edu.co
Received 07-VI-2012. Corrected 16-X-2012. Accepted 15-XI-2012.












 uBio
uBio