Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.61 n.3 San José Sep. 2013
Plant regeneration from callus cultures of Vitex trifolia (Lamiales: Lamiaceae): a potential medicinal plant
Regeneración de plantas a partir de cultivos de callos de Vitex trifolia (Lamiales: Lamiaceae): un planta medicinal potencial
Regeneración de plantas a partir de cultivos de callos de Vitex trifolia (Lamiales: Lamiaceae): un planta medicinal potencial
*Dirección para correspondencia:
Abstract
Vitex trifolia is a shrub species with popular use as a medicinal plant, for which leaves, roots and flowers have been reported to heal different distresses. The increasing exploitation of these plants has endangered its conservation, and has importantly justified the use of biotechnological tools for their propagation. Our aim was to present an efficient protocol for plant regeneration through organogenesis; and simultaneously, to analyze the genetic homogeneity of the established clonal lines by Randomly Amplified Polymorphic DNA (RAPD) and Inter Simple Sequence Repeat (ISSR) markers. Plantlet regeneration was achieved in callus cultures derived from stem, leaf and petiole explants of V. trifolia on a differently supple mented Murashige & Skoog medium, and incubated at 25±2ºC under a light intensity of 61µmol/m2s from cool white fluorescent lamps and a 16h photoperiod. The rate of shoot bud regeneration was positively correlated with the concentration of hormones in the nutrient media. Shoot buds regenerated more rapidly from stem and petiole explants as compared to leaf explants on medium containing 11.10µM BAP in combination with 0.54µMNAA. Addition of 135.74-271.50µM adenine sulphate (Ads) and 0.72-1.44µM gibberellic acid (GA3) to the culture medium increased the growth of shoot buds. The highest rate of shoot bud regeneration responses was obtained in stem explants using 11.10µM BAP in combination with 0.54µM NAA, 271.50µM Ads and 1.44µM GA3. In vitro rooting of the differentiated shoots was achieved in media containing 1.23µM indole butyric acid (IBA) with 2% (w/v) sucrose. Regenerated plantlets were successfully established in soil with 86% survival under field condition. Randomly Amplified Polymorphic DNA and Inter Simple Sequence Repeat markers analyses have confirmed the genetic uniformity of the regenerated plantlets derived from the second up to fifth subcultures. This protocol may help in mass propagation and conservation of this important medicinal plant of great therapeutic potential.
Key words: in vitro, inter simple sequence repeats, medicinal plant, micropropagation, randomly amplified polymorphic DNA, plant regeneration.
Resumen
Vitex trifolia es una especie arbustiva de uso popular como planta medicinal, sus hojas, raíces y flores se han reportado para la cura de diferentes aflicciones. El aumento de la explotación de estas plantas ha puesto en peligro su conservación y ha justificado el uso de herramientas biotecnológicas para su propagación. El objetivo de esta investigación fue presentar un protocolo eficiente para la regeneración de estas plantas a través de la organogénesis, y analizar la homogeneidad genética de las líneas clonales establecidas por ADN polimórfico amplificado aleatoriamente (RAPD) mediante la repetición de marcadores de inter secuencia simple (ISSR). La regeneración de plántulas se logró en cultivos de callos derivados de explantes de tallo, hoja y pecíolo de V. trifolia en un medio diferenciado Murashige & Skoog, que se incubaron a 25±2ºC bajo una intensidad de luz de 61μmol/m2s con lámparas fluorescentes blancas y un fotoperíodo de 16h. La tasa de regeneración de brotes se correlacionó positivamente con la concentración de las hormonas en el medio nutritivo. Los brotes se regeneraron más rápidamente a partir de explantes de tallo y pecíolos en comparación con explantes de hoja. La mayor tasa de regeneración de brotes se obtuvo en los explantes de tallo utilizando 11.10μM BAP en combinación con 0.54μM NAA, 271.50μM Ads y 1.44μM GA3. Este protocolo puede ayudar a la propagación masiva y conservación de esta importante planta medicinal de gran potencial terapéutico.
Palabras clave: in vitro, repetición inter secuencia simple, plantas medicinales, micropropagación, ADN polimórfico amplificado aleatoriamente, regeneración plantas.
The growing worldwide demand of traditional medicinal plants has made large-scale commercial cultivation and genetic improvement imperative. Vitex trifolia is one of such plants, belonging to the family verbenaceae, which has great therapeutic potential. The root is used in the treatment of painful inflammations, cough and fevers. Leaves are reported to be useful in conditions of loss of memory, loss of hair, leaucoderma and tuberculosis. Flowers are effective in treating fevers and fruits in treating amenorrhoea (Oommen et al. 2000). Besides, the fruits contain an alkaloid, vitricine which is used in treating coryza, fever, headache, photopsia, vertigo, opthalmalgia, glaucoma, rheumatism and neuralgia (Prajapati et al. 2003). Pharmaceutical companies largely depend upon material procured from naturally occurring stands which are being depleted rapidly. Thus, over exploitation concerns about possible extinction of the species, provide a significant justification for the development of in vitro propagation techniques for this crop. On the other hand, scarce availability of planting material, slow regeneration in nature and lack of cultivation practices might be the possible reasons for multiplication of this plant species by shoot organogenesis from callus cultures, an effective method for multiplication of medicinal plants (Grewal & Atal 1976, Khanna et al. 2006). Though in vitro multiplication of V. trifolia was achieved through clonal propagation through meristems culture (Hiregoudar et al. 2006), to date, there are no reports on plant regeneration via callus culture in this species.
Generally, organogenic differentiation is considered a useful method in achieving a high frequency of shoot regeneration within a short period of time. However, there is a chance in occurrence of somaclonal variations among the sub-clones of parental line. The frequency of these variations varies with the source of explants, media composition and cultural conditions (Damasco et al. 1996, Salvi et al. 2001). The cryptic genetic defects arising via somaclonal variation in the regenerants is a potential drawback when the propagation of an elite species is intended, due to uncontrol lable and unpredictable nature of variation which seriously limits the utility of the micropropagation system. A number of molecular markers can be used to assess the genetic fidelity of in vitro derived clones. However, RAPD and ISSR markers are very simple, fast, cost effective, highly discriminative and reliable, require a small quantity of DNA, and do not need any prior primer sequence information (Cassells et al. 1997, Lakshmanan et al. 2007). In this paper we report, for the first time, a rapid micropropagation protocol for V. trifolia through callus cultures and the genetic homogeneity of established clonal lines using RAPD and ISSR markers.
Materials and Methods
Plant material and surface sterilization: This study was conducted at the Directorate of Medicinal and Aromatic Plants Research (DMAPR), Anand, Gujarat, India. Shoots (5-7cm long) collected from healthy plants of V. trifolia grown at the gene bank of Directorate of Medicinal and Aromatic Plants Research, Boriavi, Anand, Gujarat, India. Shoots were washed in 2% (v/v) detergent solution ‘Teepol’ (Qualigen, India) and surface sterilized in 0.1% (w/v) aqueous mercuric chloride (HgCl2) solution for 20 minutes after rinsing 4-5 times with sterile distilled water, leaves, petioles and stem internodes were cut in to smaller segments (60x50mm, 50x40mm and ~ 0.5cm, respectively) for use as the explants.
Culture medium: The explants were placed on semi-solid (0.8% agar) basal MS medium (Murashige & Skoog 1962) supplemented with different concentrations and combinations of 6-benzylaminopurine (BAP: 0.0, 2.22, 4.44, 6.65, 8.88, 11.10, 13.32µM), kinetin (Kn: 0.0, 2.32, 4.64, 6.96, 9.28, 11.60, 13.92µM), adenine sulphate (Ads: 67.87, 135.75 and 271.50µM), 1-naphthalene acetic acid (NAA: 0.0, , 2.69, 8.06, 10.74, 13.43, 16.11µM), 2.4-dichlorophenoxy acetic acid (2.4-D: 0.0, 2.26, 4.52, 6.78, 9.04, 11.3, 13.56µM) and gibberellic acid (GA3 : 0.00, 0.29, 0.72, 1.44 µM) for callusing and organogenesis. The media pH was adjusted to 5.8 using 0.1N HCl or 0.1N NaOH before autoclaving. Routinely, 25mL of the liquid medium with 0.8% (w/v) agar (Qualigen, India) was dispensed into culture tubes (25x100mm), plugged with non-absorbent cotton and sterilized at 121ºC and 104kPa for 15min. The cultures were maintained by regular subcultures at 4-week intervals on similar fresh medium.
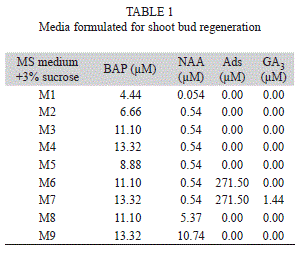
Culture condition: At the time of callus initiation, all cultures were incubated at 25±2ºC under a light intensity of 61µmol/m2s from cool white fluorescent lamps (Philips, India) under 16h photoperiod. For organogenesis, approximately, 200±10mg of fresh callus was placed in each culture tube containing different concentrations and combination of cytokinins (BAP, Kn and Ads) and auxins (NAA and 2,4-D). The different regeneration media were presented in table 1.
The cultures were incubated separately under 16 and 24h photoperiods with a light intensity of 61µmol/m2s from cool white fluorescent lamps at 25±2ºC.
Induction of rooting and acclimatization: Excised shoots (1-2cm) regenerated from the callus were cultured on rooting medium fortifying with basal MS semi-solid medium either alone or in combination with different concentrations of IBA (0.49, 0.73, 0.98, 1.23µM) or NAA (0.54, 0.81, 1.07, 1.34µM) and 2% (w/v) sucrose for root initiation. One excised shoot was cultured in each tube (25x150mm) containing 15mL of the culture media. The cultures containing different concentrations of IBA and IAA were incubated separately under 16 and 24h photoperiods with cool, white fluorescent lamps at 25±2ºC temperature. Rooted plantlets were thoroughly washed to remove the adhering gel and planted in 2.5cm earthen pots containing a sterile mixture of soil, sand and well decomposed manure in the ratio of 1:1:1 (v/v/v), and were kept in the greenhouse for acclimatization.
Observations of cultures and presentation of results: Twenty cultures were used per treatment and each experiment was repeated at least three times. The data pertaining to mean percentage of cultures responding to callusing, percentage of organogenic calli/culture, percentage of shoot buds/culture, mean percentage of rooting and number of roots/shoot were statistically analyzed by Duncan’s multiple range test. Between the treatments, the average figures followed by the same letter were not significantly different at p<0.05 (Harter 1960).
DNA extraction and PCR amplification: DNA was extracted from fresh leaves derived from both micropropagated and field-grown donor plant by the cetyltrimethyl ammonium bromide (CTAB) method (Bousquet et al. 1990) with minor modifications; 1% polyvinylpyrrolidone (PvP) was added to remove polyphenols. DNA quantity was estimated spectrophotometrically (vARIAN, Cary 300, USA) by measuring the absorbance at 260nm. Sixteen plants were assessed from which one is the donor plant and other 15 are in vitro derived plants.
The RAPD analysis was performed according to Williams et al. (1990) and ISSR analysis according to Zeitkiewicz et al. (1994). RAPD and ISSR amplifications were performed routinely using PCR mixture (25µL) containing 25ng of genomic DNA as template, 10 X PCR buffer, 200μM dNTPs (MBI Ferment Inc. USA), 1U of Taq polymerase (Bangalore Genei, India) and 15ng of RAPD primer or 40ng of ISSR primer. The amplification was carried out in a thermal cycler (S1000; BioRad, Hercules, USA). In RAPD, PCR was performed at initial temperature of 94ºC for 5min for complete denaturation. The second step consisted of 42 cycles having three ranges of temperature, i.e. 92ºC for 1min for denaturation of template DNA, 37ºC for 1min for primer annealing, 72ºC for 2min for primer extension, followed by running the samples at 72ºC for 7min for complete polymerization. For ISSR the same temperature profile was followed, but the primer annealing temperature was set at 5ºC lower than the melting temperature. The PCR products obtained from RAPD were analyzed in 1.5% agarose gel whereas the ISSR products were analyzed in 2% agarose gel. The size of the amplicons were estimated using O’ Gene RulerTM100 bpplus DNA ladder (MBIFermentas, vilnius, Lithuania) and documented in the GelDoc (Bio-Rad, Hercules, USA).
Amplified DNA markers were scored as present or absent for both the micropropagated and the mother plants. Electrophoretic DNA bands of low visual intensity that could not be readily differentiated as present or absent were considered ambiguous markers which were not scored.
Results
Callus induction: Calli were initiated from stem, petioles and leaves within 18-20 days of culture on MS basal medium supplemented with different concentrations of 2,4-D or NAA in combination with either Kn or BAP. Initially, small greenish white calli developed on the cut ends within 18-20days of inoculation which subsequently covered the entire surface of the explant (Fig. 1A). There was no sign of callus initiation in the explants cultured in only MS media devoid of cytokinins and auxins. Globular and greenish calli developed on the surface of the explants 3-4 weeks after culture initiation on MS basal medium supplemented with varying concentrations of BAP with NAA. The medium containing 2,4-D (9.05-11.3µM) and BA (0.04-0.44µM) promoted rapid callus growth from stem, petiole and leaf explants which subsequently turned brown within four weeks of culture. Similarly, also rapid callus induction was obtained in the medium supplemented with TDZ (0.45-2.27µM) + Ads (271.50µM) from the leaf and stem explants which were soft or gelatinous depending on the TDZ level; the rate of callus growth was faster in the first four-weeks of culture after which it declined. Though slow callus growth took place in media supplemented with 11.10µM BAP and 0.54- 2.70µM NAA in stem, petiole and leaf, however the best granular callus growth was observed in the medium containing 0.44µM BAP and 16.11µM NAA. However, callus morphology varied with different plant growth regulators used in the MS culture medium. The appearance of the calluses varied with all the three explant types and media. The calli derived from stem and petiole explants were invariably greener and more granular than the leaf-derived callus on the media containing 0.44 -11.10µM BAP with 0.54-16.11µM NAA. Higher concentration of NAA induced compact calli which is not desirable for plant regeneration. Media containing Kn in combination with either NAA or 2,4-D did not induce any callus growth. Of the three explant types used, early callus growth was observed in stem explants followed by petiole and leaf explants. Leaf explants produced callus at a relatively slow rate. Young leaf and mature stem explants responded better than mature leaf and young stem explants respectively (data not shown).
Differentiation of shoots from callus: After eight weeks on callus induction medium (MS+0.44µM BAP+16.11µM NAA) the calli were subcultured into media containing different concentrations of BAP, Kn and NAA for shoot bud regeneration. The calli differentiated into green nodular structures which developed into dark green shoot buds in the media supplemented with 10.0 -13.32µM BAP and 0.54µM NAA. Kinetin or BAP alone did not induce any morphogenic response, and also the combination of BAP or Kn with 2,4-D did not help in regeneration of shoot buds. The addition of adenine sulphate (271.50µM) and GA3 (1.44µM) in the culture medium, how-ever, resulted in quick growth of shoot buds within four weeks of culture (Fig. 1 B and C). A very high percentage of regeneration was observed in mature stem (86.67%) followed by petiole (76.67%) and leaf (58.33%) explants derived calli cultured in media supplemented with 13.32µM BAP+0.54µM NAA (Table 2). Regeneration of shoot buds also took place at low concentration BAP, but less frequently. With the increase of NAA concentration (5.37-10.74µM) in the medium, the rate of regeneration slowed down. The percentage of shoot bud regeneration and the frequency of regenerated shoot/culture varied from 7.25 to 16.0, 3.45 to 10.85, and 2.60 to 9.80 in stem, petiole and leaf explants, respectively (Table 1).
Among the five subcultures at four week intervals, the higher regeneration potential and shoot buds per culture were observed in the 2nd subculture. After five to six subcultures of callus, loss of organogenic potential was observed even when cultured on similar medium or modified medium (data not shown). The regeneration frequency in cultures from stem, petiole and leaf explants was found to be best under 16h photoperiod as compared to the cultures under continuous light, which was not conducive to shoot bud regeneration and multiplication.
Induction of rooting in regenerated plants: Microshoots regenerated from stem and petiole derived calli were excised and transferred to the medium with or without growth regulators. Half strength MS medium without growth regulators did not promote root induction. Roots were formed on excised shoots grown in ½ MS media containing either IBA (0.49-1.23µM) or NAA (0.54-1.34µM) with 2% (w/v) sucrose. A high percentage of shoots (86%) rooted in the medium containing half strength MS basal salts with 1.23µM IBA+2% (w/v) sucrose after 11-12days of culture (Table 3). Root initials formed within 7-9 days which developed a good root system in 11-12 days (Fig. 1 D). Root initiation was achieved on medium containing either NAA (1.23µM) or IAA (1.43µM) but with intervening callus at the cut end of the shoot. The rooting of microshoots was observed to be best under 16h irradiance which was found to be inhibited in continuous light.
Acclimatization of rooted plants: Rooted plantlets were transferred into pots containing soil: sand: well decomposed cow-dung manure, in the ratio of 1:1:1 and kept in the green house for acclimatization. After one month of transfer to soil, about 90% of the plants survived in the green house (Fig. 1 E) and were subsequently planted in the field. No morphological variation was noticed.
Clonal fidelity: DNA samples from the hardened plantlets of in vitro-grown shoots derived from second up to fifth cycle of shoot multiplication grown in the polyhouse and the donor plants were subjected to RAPD and ISSR analysis. A total of 40 plantlets were analyzed taking a minimum 15 plants from each culture period along with the donor plant. For RAPD analysis, a total of 60 random primers were used out of which 20 random decamer primers (Operon Technologies, Almeda, USA) were selected. In the case of ISSR, 12 out of 27 primers were selected. A total of 20 selected RAPD primers gave rise to a total of 125 scorable bands ranging from 180 to 3 000bp from which two markers (OPA & OPN 04) showing monomorphic bands are represented in figure 2 (A and B).The number of bands for each primer varied from 4-9 with an average of 6.25 bands per primer. The highest number of bands (9) was obtained with primer OPA10 and the lowest number of bands (4) amplified in OPA 05, OPA 19 and OPP 10 (Table 4). In ISSR analysis, 12 selected primers (Bangalore Genei, India) produced a total of 49 scorable bands ranging from 400 to 2 000bp (Table 5). For each primer, the number of amplified bands varied from 1-7, and a total of 49 bands were generated all of which were found to be monomorphic in nature; a common fragment size (400-1 220bp) amplified with two primers such as (AG)8C and (AC)8C are represented in figure 3 (A and B). The highest number of bands (7) and the lowest number of bands (1) were generated with the primer (CA)6GG and (CT)8T, respectively.
Discussion
The present study demonstrated the possibility of exploring the morphogenetic potential in callus derived from stem, petioles and leaves of V. trifolia with the application of various growth regulators. The importance of auxin and cytokinin balance has been reported in regulating the apical dominance as well as morphogenetic events such as shoot formation and multiplication (Li & Bangerth 2003, Werner et al. 2003, Nakagawa et al. 2005). Also, another factor that may influence the morphogenetic events during in vitro culture is a possible interaction between the endogenous concentrations of plant growth regulators and those present in the culture media (Mercier et al. 2003). Our results showed that BAP was more effective for shoot bud proliferation than kinetin also corroborated in Samantaray & Maiti (2008). Moreover, the concentrations 11.10-13.32µM of BAP in the medium favored the shoot bud proliferation and multiplication, which could be the optimum BAP concentration for cytokinin and endogenous auxin balance resulting in shoot bud regeneration. However, in the present study, combination of high concentrations of BAP with low concentrations of NAA enhanced the shoot bud multiplication as reported earlier (Prakash & Staden 2008, Amoos et al. 2009). The usefulness of different combinations of auxin and cytokinin for the production of organogenic and subsequent plant regeneration was well documented (Irvani et al. 2010, Bantawa 2011). Besides, the combination of cytokinins and auxins sometimes triggered the rate of shoot bud regeneration in various medicinal plants (Sivanesan & Jeong 2007, Samantaray & Maiti 2008, Samantaray et al. 2009).
Though BAP and NAA played a significant role in shoot bud regeneration, however, addition of adenine sulphate and GA3 in the culture medium resulted in quick growth of shoot buds within four weeks of culture which is corroborated with the findings of earlier reports (Mohan & Krishnamurthy 1998, Samantaray & Maiti 2010). The percentage of shoot bud regeneration and the frequency of regenerated shoot/culture varied significantly in stem, petiole and leaf explants. The use of various explants for the production of callus and subsequent regeneration of shoot buds was well documented (Arora & Bhojwani 1989, Cacho et al. 1991). The differential response could be due to the growth regulators in the medium and explant types (Cacho et al. 1991, Pellegrineschi & Tepfer 1981). Differentiation of callus into green nodular structures followed by dark shoot buds were achieved in the media supplemented with high concentration of BAP and low concentration of NAA as reported earlier (Saxena et al. 1997, Samantaray & Maiti 2011). The regeneration frequency in cultures from stem, petiole and leaf explants was found to be best under 16h photoperiod as compared to the cultures under continuous light which was not conducive to shoot bud regeneration and multiplication. Similar effects of photoperiod on shoot bud regeneration were observed in Sesbania rostrata (Perez-Bermudez et al. 1984), Actinidia deliciosa (Muleo & Morini 1990), Lavandula latifolia (Calvo & Segura 1989) and Trema orientalis (Samantaray et al. 1995).
Optimal rooting and growth of microshoots were observed on medium containing low concentration of IBA without intervening callus. Similar observations were made in various medicinal plants such as Vitex agnuscastus, Filipendula ulmaria, Chlorophytum arundiaceum (Balaraju et al. 2008, Yildirim & Turker 2009, Samantaray & Maiti 2011). On the other hand, the irradiance showed significant effects on rooting induction (Sivanesan & Jeong 2007). The rooting in microshoots performed better under 16h photoperiods compared to continuous light. The rate of rooting dependent on growth regulators and photoperiod was also well documented (Murashige 1974, Rout et al. 1995).
The occurrence of genetic defects arising from variation in the regenerants seriously affects the true-to-type nature of the plants, which in turn hampers the production of a specific secondary metabolite of commercial value. Hence, the production of genetic uniform and stable plants is a prerequisite for commercial purposes (Shu et al. 2003). Though many PCR-based techniques such as SSR, RAPD and AFLP have been used to determine somaclonal variation of regenerants, RAPD and ISSR markers were successfully employed for the detection of variations at the genome level among tissue cultured regenerants (Taylor et al. 1995, Choudhuri et al. 2009). The variations can be due to gene amplification, chromosomal irregularities, point mutation and alteration in DNA methylation during in vitro culture (Saker et al. 2000). In the present study, amplification of genomic DNA of the arbitrarily selected in vitro derived progenies and that of the donor plant of V. trifolia was similar which indicates the true-to-type nature of the progenies (Samantaray & Maiti 2008, 2010).
In conclusion, we established a suitable regeneration system protocol for V. trifolia, a potential medicinal plant, via organogenesis using three different explants viz. stem, leaf and petiole which may be very much useful for mass propagation and genetic transformation. Our results indicate the organogenic potential of V. trifolia is greatly influenced by NAA and BABesides, the efficient usefulness of RAPD and ISSR techniques in assessing the truetotype nature of the regenerated clones of V. trifolia was confirmed.
Acknowledgments
The authors wish to acknowledge the help of the Directorate of Medicinal and Aromatic Plants Research, Boriavi, Anand for providing necessary facilities.
References
Amoos, O., J.F. Finnie & J. van Staden. 2009. In vitro propagation of Huernia hystrix: an endangered medicinal and ornamental succulent. Plant Cell Tiss. Org. Cult. 96: 273-278.
[ Links ]
Arora, R. & S.S. Bhojwani. 1989. In vitro propagation and low temperature storage of Saussurea lappa C.B. ClarkeAn endangered plant. Plant Cell Rep. 8: 44-47. [ Links ]
Balaraju, K., P. Agastian, J.P. Preetamraj, S. Arokiyaraj & S. Ignacimuthu. 2008. Micropropagation of Vitex agnus-castus (verbenaceae)- A valuable medicinal plant. In Vitro Cell. Dev. Biol. Pl. 44: 436-441. [ Links ]
Bantawa, P., O. Saha-Roy, S.K. Ghosh & T.K. Mondal. 2011. In vitro regeneration of an endangered medicinal plant Picrorhizas crophulariiflora. Biol. Plantar. 55: 169-172. [ Links ]
Bousquet, J., L. Simon & M. Lalonde. 1990. DNA amplification from vegetative and sexual tissues of trees using polymerase chain reaction. Can. J. Forest Res. 20: 254-257. [ Links ]
Cacho, M., M. Moran, M.T. Herriera & J. Fernandez- Tarrago. 1991. Morphogenesis in leaf, hypocotyl and root explants of Digitalis thapsi L. cultured in vitro. Plant Cell Tiss. Org. Cult. 25: 117-123. [ Links ]
Cassells, A.C., J.T. Croke & B.M. Doyie. 1997. Evaluation of image analysis, flow cytometry and RAPD analysis for the assessment of somaclonal variation and induced mutation in tissue culture-derived Pelargonium plants. Angew. Bot. 71: 125-130. [ Links ]
Calvo, M.C. & J. Segura. 1989. Plant regeneration from cultured leaves of Lavandula latifolia Medicus: Influence of growth regulators and illumination conditions. Plant Cell Tiss. Org. Cult. 19: 33-42. [ Links ]
Choudhuri, R.K., A. Pal & T.B. Jha. 2009. Regeneration and characterization of Swertia chirata Buch.-Ham. ex Wall. plants from immature seed cultures. Scientia Hort. 120: 107-114. [ Links ]
Damasco, O.P., G.C. Graham, R.J. Henry, S.W. Adkins, M.K. Smith & I.D. Godwin. 1996. Random amplified polymorphic DNA (RAPD) detection of dwarf off types in micropropagated Cavendish (Musa spp. AAA) bananas. Plant Cell Rep. 16: 118-123. [ Links ]
Grewal, S. & C.K. Atal. 1976. Plantlet formation in callus cultures of Dioscorea deltoidea Wall. Indian J.exp. Biol. 14: 352-353. [ Links ]
Harter, H.L. 1960. Critical value for Duncan’s multiple range test. Biometrics 16: 671-685. [ Links ]
Hiregoudar, L.v., H.N. Murthy, J.G. Bhat, A. Nayeem, B.P. Hema, E.J. Hahn & K.Y. Paek. 2006. Rapid clonal propagation of Vitex trifolia. Biol. Plantar. 50: 291-294. [ Links ]
Irvani, N., M. Solouki, M. Omidi, A.R. Zare & S. Shahnazi. 2010. Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tiss. Org. Cult. 100: 293-299. [ Links ]
Khanna, P.K., A. Ahuja, M. Sharada, G. Ram, K. Koul & M.K. Kaul. 2006. Regeneration via organogenesis in callus cultures of Argyrolobium roseum. Biol. Plantar. 50: 417-420. [ Links ]
Lakshmanan, v., S.R. venkataramareddy & B. Neelwarne. 2007. Molecular analysis of genetic stability in long term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotechnol. 10: 1-8. [ Links ]
Li, C. & H. Bangerth. 2003. Stimulatory effect of Cytokinins and interaction with IAA on the release of lateral buds of pea plants from apical dominance. J. Plant Physiol. 160: 1059-1063. [ Links ]
Mercier, H., B.M. Souza, J.E. Kraus, R.M. Hamasaki & B. Sotta. 2003. Endogenous Auxin and Cytokinin contents associated with shoot formation in leaves of pineapple cultured in vitro. Braz. J. Plant Physiol. 15: 107-112. [ Links ]
Mohan, M.L. & K.v. Krishnamurthy. 1998. Plant regeneration in pigeon pea [Cajanus cajan (L.) Millsp]. Plant Cell Rep. 17: 705-710. [ Links ]
Muleo, R. & S. Morini.1990. Effect of light quality on regeneration from callus of Actinidia deliciosa. Acta Hortic. 280: 155-158. [ Links ]
Murashige, T. 1974. Plant propagation through tissue culture. Annu. Rev. Plant Phys. 25: 135-166. [ Links ]
Murashige, T. & F. Skoog. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plantar. 15: 473-497. [ Links ]
Nakagawa, H., C.J. Jiang, H. Sakakibara, M. Kojima, I. Honda, H. Ajisaka, T. Nishijima, M. Koshioka, T. Homma, L.N. Mander & H. Takatsuji. 2005. Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. Plant J. 41: 512-523. [ Links ]
Oommen, S., D.K. ved & R. Krishnan. 2000. Tropical Indian Medicinal Plants-Propagation methods, Foundation for Revitalization of Local Health Tradition, Bangalore, India. [ Links ]
Pellegrineschi, A. & D. Tepfer. 1981. Micropropagation and plant regeneration in Sesbania rostrata. Plant Sci. 88: 113-119. [ Links ]
Perez-Bermudez, P., M.C. Brisa, M.J. Cornezo & J. Segura. 1984. In vitro morphogenesis from excised leaf explants of Digitalis obscura L. Plant Cell Rep. 3: 8-9. [ Links ]
Prajapati, N.D., S.S. Purohit, A.K. Sharma & T. Kumar. 2003. A Handbook of Medicinal Plants - A Complete Source book. Agrobios, Jodhpur, India. [ Links ]
Prakash, S. & J.v. Staden. 2008. Micropropagation of Searsia dentate. In Vitro Cell Dev. Biol. Pl. 44: 338-341. [ Links ]
Rout, G.R., S. Samantaray & P. Das. 1995. Regeneration of Trema orientalis (Blume) Linn.: Effect of growth regulators, culture conditions, age and source of explants. Israel J. Plant Sci. 43: 391-395. [ Links ]
Saker, M.M., S.A. Bekheer, H.S. Taha, A.S. Fahmy & H.A. Moursy. 2000. Detection of somaclonal variations in tissue cultured-derived date palm plants using iso-enzyme analysis and RAPD fingerprints. Biol. Plantar. 43: 347-351. [ Links ]
Salvi, N.D., L. George & S. Eapen. 2001. Plant regeneration from leaf callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tiss. Org. Cult. 66: 113-119. [ Links ]
Samantaray, S., S.v. Kumar & S. Maiti. 2009. Direct shoot regeneration from immature inflorescence cultures of Chlorophytum arundinaceum and Chlorophytum borivilianum. Biologia 64: 305-309. [ Links ]
Samantaray, S. & S. Maiti. 2008. Rapid plant regeneration and assessment of genetic fidelity of in vitro raised plants in Aloe barbadensis Mill. using RAPD markers. Acta Bot. Gallica 155: 427-434. [ Links ]
Samantaray, S. & S. Maiti. 2010. An assessment of genetic fidelity of micropropagated plants of Chlorophytum borivilianum using RAPD markers. Biol. Plantar. 54: 334-338. [ Links ]
Samantaray, S. & S. Maiti. 2011. Factors influencing rapid clonal propagation of Chlorophytum arundinaceum (Liliales: Liliaceae) - an endangered medicinal plant. Int. J. Trop. Biol. 59: 1-11. [ Links ]
Samantaray, S., G.R. Rout & P. Das. 1995. An in vitro study on organogenesis in Trema orientalis (Blume) Linn. Plant Sci. 105: 87-94. [ Links ]
Saxena, C., S.K. Palai, S. Samantaray, G.R. Rout & P. Das. 1997. Plant regeneration from callus cultures of Psoralea corylifolia Linn. Plant Growth Regul. 22: 13-17. [ Links ]
Shu, Q.Y., G.S. Liu, D.M. Qi, C.C. Chu, J. Liu & H.J. Li. 2003. An effective method for axillary bud culture and RAPD analysis of cloned plants in tetraploid black locust. Plant Cell Rep. 22: 175-180. [ Links ]
Sivanesan, I. & B.R. Jeong. 2007. Direct shoot regeneration from nodal explants of Sidacordifolia Linn. In Vitro Cell Dev. Biol. Pl. 43: 436-441. [ Links ]
Taylor, P.W.J., J.R. Geijskes, H.L. Ko, T.A. Fraser, R.J. Henry & R.G. Birch. 1995. Sensitivity of random amplified polymorphic DNA analysis to detect genetic change in sugar cane during tissue culture. Theor. Appl. Genet. 90: 1169-1173. [ Links ]
Werner, T., v. Motyka, v. Laucou, R. Smets, H. van Onckelen & T. Schmulling. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532-2550. [ Links ]
Williams, J.G.K., A.R. Kubelik, K.J. Liva, J.A. Rafalski & Tingey S.v. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acids Res. 18: 6531-6535. [ Links ]
Yildirim, A.B. & A.U. Turker. 2009. In vitro adventitious shoot regeneration of the medicinal plant meadowsweet Filipendula ulmaria (L.) Maxim. In vitro Cell Dev. Biol. Pl. 45: 135-144. [ Links ]
Zeitkiewicz, E., A. Rafalski & D. Labuda. 1994. Genome finger printing by simple sequence repeat (SSR)- anchored PCR amplification. Genomics 20: 176-183. [ Links ]
*Correspondencia a:
Sanghamitra Samantaray. Central Rice Research Institute (CRRI). Correspondence.
Ashok Kumar Bishoyi. Cuttack753006, Orissa, India; smitraray@gmail.com, smitralok@rediffmail.com
Satyabrata Maiti. Cuttack753006, Orissa, India; smitraray@gmail.com, smitralok@rediffmail.com
1. Central Rice Research Institute (CRRI). Correspondence
2. Cuttack753006, Orissa, India; smitraray@gmail.com, smitralok@rediffmail.com
3. Directorate of Medicinal and Aromatic Plants Research Boriavi, Anad-387310, Gujarat, India; ashokbiotech4@gmail.com, satyabratamaiti@hotmail.com
Received 06-VI-2012. Corrected 08-XI-2012. Accepted 13-XII-2012