Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.58 n.3 San José Sep. 2010
Reproduction of the fish Lutjanus analis (mutton snapper; Perciformes: Lutjanidae) from Northeastern Brazil
Simone F. Teixeira1, Yalan F. Duarte2 & Beatrice P. Ferreira2
1. Universidade de Pernambuco, Laboratório de Ecologia de Peixes Tropicais, Rua Arnóbio Marques, 310, Recife/PE, Brasil, 50.100-130; teixeirasf@ibest.com.br
2. Universidade Federal de Pernambuco, Laboratório de Nectologia e Aqüicultura, Av. Arquitetura, s/n, Recife/PE, Brasil, 50.670-901; yalanf@yahoo.com, beatrice@ufpe.br
Abstract: The mutton snapper Lutjanus analis is an overexploited species of economic importance for the Northeast region of Brazil. To determine the reproductive aspects of the mutton snapper, biological data were sampled from Bahia, Pernambuco, Paraíba, Rio Grande do Norte and Ceará States, between 7 and 200m depth, from February 1997 to December 1999, performed in the Brazilian Economic Exclusive Zone Study Program (REVIZEE-NE Program). Data on total length (TL), fork length (FL), standard length (SL), total weight (TW) and gutted weight (GW) were measured. The gonads were collected and fixed in FAACC for histological analysis on maturational stage and calculation of the gonadosomatic index (GSI). A total of 135 fishes were collected; from these, 74 were females and 61 were males. Females were between 28.0 and 69.0cm (TL) and the males between 35.0 and 75.0cm (TL). Mutton snapper are gonochorists, and the spawning season, determined by the maturity stages frequency and the GSI, occurred between November and April, with peak spawning in March. The mature females’ minimum length was 28.0cm fork length, smaller than the minimum maturation length previously reported for the species in Cuba. The exploitation status of the species and the biological traits enhances the importance of establishing spatial and seasonal protection measures such as protected areas both in nursery and spawning sites. Rev. Biol. Trop. 58 (3): 791-800. Epub 2010 September 01.
Key words: L. analis, sexual structure, spawning mode, spawning season, Northeastern Brazil.
Lutjanids generally live in deep-rocky or reef environments and constitute a commercially important fishery resource due to its abundance and market quality. Snapper fishing in the Brazilian Northeast occurs from the coastal waters to the continental slope, banks and oceanic islands (Resende et al. 2003). Twelve lutjanids species, including mutton snapper Lutjanus analis (Cuvier 1828), are caught representing about 12.5% of the total commercial landings controlled in the Ceará, Rio Grande do Norte and Pernambuco States (Resende et al. 2003).
Snappers are caught using hook and line, compressor assisted spear fishing, and even longlines. They are generally caught over the continental shelf or oceanic banks, between 7 and 200m depth. The mutton snapper, in the Brazilian Northeast, is mainly caught by hook and line with optimal catches occurring between 20 and 80m depth (Frédou & Ferreira 2005).
Lutjanids are considered gonochoristic, achieving sexual maturity with 40 to 50% of the maximum length and fecundity values are high (Grimes 1987). Spawning is assumed to be nocturnal (Grimes 1987), but in the Dry Tortugas three or four spawning events of mutton snapper have been observed all around 4:30 p.m. (M.L. Burton 2009, pers. comm.). In Brazil, no spawning aggregations of the mutton snapper have been recorded to date, however anecdotal reports of large schools of the cubera snapper Lutjanus cyanopterus are not uncommon (Gerhardingher et al. 2009) so it is possible that similar events also occur for the mutton snapper as reported in other parts of the Atlantic. Some abiotic and biotic factors such as temperature, photoperiod, nutrients, primary production and feeding, may influence their reproduction (Grimes 1987). Snapper displays two reproductive seasonality patterns, irrespective of latitude; continental populations and species exhibit summer-extended spawning while insular populations and species exhibiting protracted spawning with spring and fall peaks (Grimes 1987). Mutton snapper, at thirteen reproductive places on the Cuban shelf spawning, depending on the sites, varied between April to September, but peak spawning at all sites was in May or June (Claro & Lindeman 2003).
Mutton snapper, specie of great economic importance for the Northeast region of Brazil, is currently on Brazil´s list of overexploited species or under risk of overexploitation (Brasil 2004) and is globally classified as vulnerable by the IUCN (International Union for Conservation of Nature) (IUCN 2009). Our objective in this study is to contribute to improve the management of mutton snapper caught by the artisanal fishing fleet of Northeast Brazil, providing information about the sex structure, gonadal development, spawning mode and seasonality of reproduction.
Materials and methods
We sampled landings of artisanal and commercial fleets in the States of Bahia, Pernambuco, Paraíba, Rio Grande do Norte and Ceará, from February 1997 to December 1999, supported by the REVIZEE (Brazilian Economic Exclusive Zone Study Program)/Score-NE (Fig. 1). Additionally, data from research expeditions carried out on board of the Ship R/V "Martins Filho" and Fishing Vessel "Reinaldo", using bottom longline and traps on the slope and reef banks of the Northeast, were also used.
The following data were obtained for all individuals: sex; total length (TL), fork length (FL) and standard length (SL) (±0.1cm); total weight (TW), gutted weight (GW) (±0.1kg); and gonadal total weight (GTW) (±0.1g).
We used regressions between lengths (FL vs. TL and FL vs. SL) and fork length and gutted weight to estimate the fork length of fishes that were disfigured by the application of identification marks by fishermen. The regression Y=a+bx was used for the FL–TL and FL– SL regressions and Y=axb was used for length and weight regression, with significance level of 5% (Zar 1996).
Population sex structure was analyzed in order to determine the sex ratio by size classes and by month. A chi-square analysis (Zar 1996) was applied, assuming an expected proportion of 1:1 between sexes.
Gonads collected from 135 fishes were immediately fixed in FAACC (Ferreira 1993) for histological processing. Gonads were embedded in paraffin, sectioned transversally at 6 to 8μm and stained with haematoxylineosin.
Histological description of the gonads and stages of maturity were obtained using the middle part of the gonads sampled. The spawning mode was determined by microscopic observation of the gonads, in order to classify females and males on ontogenetic stages and gonadal development. The oogenesis and gonadal stages followed the description of Vazzoler (1996). The classification on the males’gonadal stages followed Crim & Glebe (1990). Stages of spermatogenesis followed the description of Silveira et al. (1995).
The spawning season was determined through the temporal variation of the frequency of the gonadal stages and by the temporal variation of the gonadosomatic index (GSI). GSI was calculated as: GSI=(GTW x 100)/GW, where GTW is the gonadal total weight (g) and GW is the gutted weight (g).
Results
Sexual structure: From the 135 gonads analyzed 61 were males and 74 were females (Table 1). Sex ratio, by length class and month, are showed in Figures 2 and 3, respectively. Females dominated the size classes below 40.0cm, with a 28.0cm FL female (the smallest fish caught) (X2 35cm=21.78, X2 40cm=4.73, p<0.05), while there was significant presence of males in the classes over 65.0cm FL, with the largest fish observed being a male of 75.0cm (X2 65cm=4.00, X2 70cm=11.11, p<0.05) (Fig. 2).
Analysis of monthly sex ratio indicate a predominance of females in almost all months analyzed, having a significant difference between sexes only during the spawning season in December (X2=10.24, p<0.05) (Fig. 3).
Male-female sex ratio, for the entire study period, was 1:1.21 and was not significantly different (X2=0.92, n=135, p>0.05) from expected ratio 1:1.
Females: The females analyzed exhibited four different gonadal stages: early maturation, ripe, spent and resting. Early maturation is characterized by thin gonadal wall, with well organized ovarian lamellae. The ovary presents projections of the gonadal wall for its interior promoting sustainability to inner lamellae. Inside the lamellae are oocytes in different stages of pre-vitellogenic growth (gonium, chromatin nucleolus and perinucleolus stage). A presence of rare oocytes on yolk globule stage was also observed (Fig. 4A). During ripe the gonadal wall is reduced, lamellae is well organized and inside there are the oocytes in pre-vitellogenic and vitellogenic growth with higher predominance on the yolk globule stage. Hydrated oocytes were also observed (Fig. 4B). In spent gonadal wall is quite slim, with lamellae totally disorganized and with oocytes in pre-vitellogenic and vitellogenic growth presenting a higher number and residual oocytes in vitellogenic growth in reabsorption. Brown bodies were observed in some gonads (Fig. 4C); and resting the gonadal wall is slim, with well organized ovarian lamellae, inside the lamellae there were oocytes in pre-vitellogenic growth.
The presence of oocytes, from oogonium to hydrated oocytes, was observed on the histological analyses, characterizing multiple spawning, and the monthly distribution of gonadal stages indicated higher percentage of females on ripe and spent through November to April (Fig. 5), a second spawning is in June and July. The gonadosomatic index (GSI) increase in November, presented spawning peak in March, and started to decline in April, remaining low through October (Fig. 6).
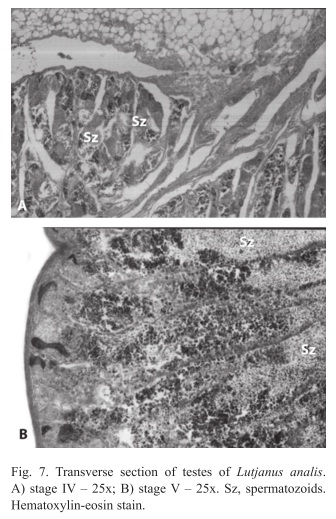
Males: We found four different gonadal stages (III, IV, V and VI). During stage III gonadal wall are reduced, with organized seminiferous lobules, constituted by cells on stages of spermatogonia, primary and secondary spermatocytes and spermatids. More spermatid cells were observed on the seminiferous lobule. In stage IV gonadal wall are thin with organized seminiferous lobules, presence of cells on stage of spermatogonia, primary and secondary spermatocytes, spermatids and few spermatozoids. More spermatid cells than in the stage III were observed on the seminiferous lobule (Fig. 7A). In stage V gonadal wall are quite slim with slightly organized seminiferous lobules, presence of cells on stage of spermatogonia, primary and secondary spermatocytes, spermatids and spermatozoids. Spermatozoids were found in the whole lobule (Fig. 7B); and in the stage VI the gonadal wall have quite organized seminiferous lobules, constituted by all stages cells, predominating the spermatogonians. Interstitial cells, blood vases and Sertoli cells were observed in the testes.
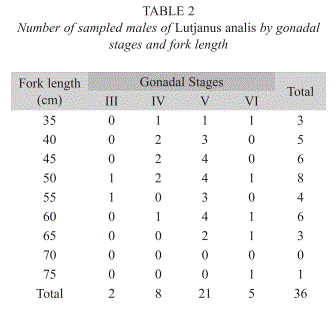
Data on the males’ gonadal stages by fork length classes are presented in the Table 2. The monthly distribution of gonadal stages indicated higher males’ percentage on stages III, IV, V and VI from November to July. Spawning season is quite long, extending from summer to half winter, and occurs between November and July.
Discussion
The male-female sex ratio for the entire study period was 1:1.21. This was not significantly different from the ratio expected for the family Lutjanidae, which is 1:1 (Garcia-Cagide et al. 2001). Smaller length classes were dominated by females, while larger length classes were dominated by males. For lutjanids the size dependent patterns of sex ratio indicates that females are more abundant in almost all length classes and reach a larger size than do males (Garcia-Cagide et al. 2001). The predominance of females among more individuals is probably a result of different longevities, as females seem to have higher longevities than males, as seen for some species of the family Lutjanidae, including Rhomboplites aurorubens (Grimes & Huntsman 1980). This pattern was not observed in this study for L. analis but according to Grimes (1987) population sex ratio, as well as sex-specific size frequencies, is often variable, likely due to the differential growth and mortality between sexes.
Histological analyses revealed females in early maturation stages up to resting and, for males, stages III and VI were observed. The fact that several oocyte developmental stages had been found in a single female’s gonads (early maturation, ripe and spent) indicates typical gonadal development of multiple spawning species. In this spawning mode, several portions of oocytes develop successive maturation stages and are spawned at different times, within the same spawning season (Vazzoler 1996). Many lutjanid species studied are multiple spawners, such as Lutjanus fulviflamma (Kaunda-Arara & Ntiba 1997), Lutjanus peru (Santamaría-Miranda et al. 2003) and Lutjanus synagris (Sousa Jr. et al. 2008). This mode of spawning, according to Nikolsky (1963) allows that the females of a same population, which reproduce in the same period, avoid competition by spawning place, beyond to guarantee larvae survival.
Traditionally, in reproductive studies, the testes are less studied than ovaries, as females are considered responsible for spawning season. As the cellular changes in spermatogenesis are not as marked as in oogenesis, it is more difficult to establish the stages of the reproductive cycle (Silveira et al. 1995). In this study, the presence of spermatozoids in all months of the year was observed in most of male gonads and concerning the immature individuals, these were not observed in either female or male. This fact may be related to the characteristics of the commercial fishing gear, which likely targets larger individuals. This may explain why smaller individuals are not harvested by these fisheries.
We observed that all females larger than 28cm fork length and all males larger than 35cm fork length were matured, with gonadal evidence of previous spawning. In Cuba, for L. analis, 41cm fork length was the minimum maturation size (Claro 1981). The minimum size of maturity observed for the mutton snapper in our study is smaller than that from Cuba, perhaps suggesting an effect resulting from fishing pressure. Although fisheries in different regions may also target different parts of the population, and thus result in differences in size based parameters, as across continental shelf and upper slope size distribution has been reported for this species (Frédou and Ferreira 2005).
The spawning season of mutton snapper, determined by using gonadal developmental stages and female GSI, occurred between November and April, with secondary spawning between June and July. Erdman (1977) found NE Caribbean mutton snapper spawned in March, and Claro (1981) determined spawning in Cuban waters occurred from March to September, peaking in June.
Claro and Lindeman (2003) found minor regional differences in the timing of spawning in Cuban waters. Spawning occurred from May to August in the Southeast region of Bajo Mandinga and Cabo Cruz; in the Southwest region spawning occurred from April to August (C. San Felipe and Cabo Corrientes) and from April to September (Banco de Jagua, Puntalón de Cayo Guano and C. Avalos); in the Northeast region mutton snapper spawned from Mayto August (Cabo Santo Antonio and Corona de San Carlos); and in the North-central region of the island, the spawning season occurred from May to August (Punta Hicacos‑Cayo Mono, C. Mégano de Nicolao and C. Caimán Grande) and from May to September (C. Sabinal). Peak spawning occurred in May and June at all sites.
Some lutjanids species show a seasonal spawning season (Kritzer 2004, Grandcourt et al. 2006) and others an extended discontinuous spawning period (Kaunda-Arara and Ntiba 1997, Rojas 1997), however, there being a preference by many species for some periods of the year (Sadovy 1996, Rojas et al. 2009), as observed in L. analis. The mutton snapper from Brazilian Northeast seems to follow the spawning pattern with extended spawning and a spawning peak during the reproductive period.
Reproduction is occurring at smaller sizes than in other areas, and is possible that overfishing is affecting growth and reproduction of this population. Therefore, the adoption of conservation steps is suggested in order to avoid a future collapse of the specie. Although no spawning aggregations of the mutton snapper have been recorded to date, evidence from other species (Gerhardingher et al. 2009) and fishers reports (B.P. Ferreira, pers. observ.) indicate that such aggregation may also occur for other snappers and be targeted by the fisheries. This suggests a large scope for the investigation of reef fish spawning aggregation in Brazil.
According to Frédou et al. (2009), who conducted fishery stock assessment for the snappers species, the conservative biological reference point F0.1 indicated an imminent need for a drastic reduction in fishing mortality for L. analis, ranging from 80% to 90%, and noted that although quite high, these results were in agreement with previous assessments of lutjanids such as the ones conducted by Newman et al. (2000). These species are indeed highly vulnerable due to their life history traits, which led the American Fisheries Society to recommend that these stocks be exploited with fishing mortalities similar to the natural mortality (Coleman et al. 2000).
The exploitation status of the species and the biological traits enhances the importance of establishing spatial and seasonal protection measures such as protected areas both in nursery and spawning sites.
Acknowledgments
We thank all the people who assisted in the fieldwork and the many commercial fishermen that allowed sampling of the mutton snapper during landings. We also appreciate and thank the anonymous referees who broadened the literature survey. This work was funded by grants from the Brazilian Ministry of Education (CNPq) and Brazilian Economic Exclusive Zone Programme - Score/NE.
Resumen
El pargo criollo Lutjanus analis es una especie de importancia económica para la región noreste de Brasil, que esta siendo sobreexplotada. Para determinar sus aspectos reproductivos, se tomaron datos biológicos en los Estados de Bahía, Pernambuco, Paraíba, Rio Grande do Norte y Ceará, entre 7 y 200m de profundidad, de febrero 1997 a diciembre 1999, en el Programa de REVIZEE-NE en la Zona Económica Exclusiva de Brasil. Se midieron la longitud total (LT), longitud de horquilla (FL), longitud estándar (SL), el peso total (TW) y peso evicerado (GW). Las gónadas se recolectaron y fijaron en FAACC para el análisis histológico de las etapas de maduración y el cálculo del índice gonadosomático (IGS). Se recolectaron un total de 135 hembras y 61 machos. Las hembras midieron 28.0-69.0cm (TL) y los machos entre 35.0-75.0cm (TL). Lutjanus analis es gonocorista, y la época de desove, determinada por la frecuencia de las etapas de maduración y el GSI, ocurre entre noviembre y abril, con con un pico de desove en marzo. La longitud mínima (FL) de las hembras maduras fue 28.0cm, menor que la reportada para la especie en Cuba. El estado de explotación de las especies y los indicadores biológicos observados justifican el establecimiento de medidas de protección espacial y temporal, como las áreas protegidas, tanto en sitios de crianza como en zonas de desove.
Palabras clave: L. analis, estructura sexual, modo de reproducción, temporada de desove, Noreste de Brasil.
Received 09-VIII-2009. Corrected 15-I-2010. Accepted 11-II-2010.
References
Brasil.Instrução Normativa no. 5, de 21 de maio de 2004. 2004. Estabelece a lista das espécies ameaçadas de extinção e espécies sobreexplotadas ou ameaçadas de sobreexplotação, p. 136-142. In Diário Oficial [da República Federativa do Brasil], n. 102. Brasília, DF, Brasil. [ Links ]
Claro, R. & K.C. Lindeman. 2003. Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf Carib. Res. 14: 91-106. [ Links ]
Claro, R. 1981. Ecología y ciclo de vida del pargo criollo, Lutjanus analis (Cuvier), en la plataforma cubana. Inf. Cient. Téc. Biol. Pesq. Acad. Cienc. Cuba 186: 1-8. [ Links ]
Coleman, F.C., C.C. Koenig, G.R. Huntsman, J.A. Musick, A.M. Eklund, J.C. McGovern, R.W. Chapman, G.R. Sedberry & C.B. Grimes. 2000. Long-lived reef fishes: the grouper-snapper complex. Fisheries 25: 14-21. [ Links ]
Crim, L.W. & B.D. Glebe. 1990. Reproduction, p. 529-553. In C.B. Schreck & P.B. Moyle (eds.). Methods for fish biology. American Fisheries Society, Bethesda, USA. [ Links ]
Erdman, D.S. 1977. Spawning patterns of fish from the North-eastern Caribbean. FAO Inf. Pesca. 200: 145- 170. [ Links ]
Ferreira, B.P. 1993. Reproduction of the inshore coral trout Plectropomus maculatus (Perciformes: Serranidae) from the Central Great Barrier Reef, Australia. J. Fish Biol. 42: 831-844. [ Links ]
Frédou, T. & B.P. Ferreira. 2005. Bathymetric trends of Northeastern Brazilian snappers (Pisces, Lutjanidae): implications for the reef fishery dynamic. Braz. Arch. Biol. Tech. 48: 787-800. [ Links ]
Frédou, T., B.P. Ferreira & Y. Letourneur. 2009. Assessing the stocks of the primary snappers caught in Northeastern Brazilian reef systems. 1-Traditional modeling approaches. Fish. Res. 99: 90-96. [ Links ]
García-Cagide, A., R. Claro & B.V. Koshelev. 2001. Reproductive patterns of fishes of the Cuban shelf, p. 73-114. In R. Claro, K.C. Lindeman & L.R. Parenti (eds.). Ecology of the marine fishes of Cuba. Smithsonian Institution, Washington D.C., USA. [ Links ]
Gerhardingher, L.C., M. Hostim-Silva, R.P. Medeiros, J. Matarezi, Á.A. Bertoncini, M.O. Freitas & B.P. Ferreira. 2009. Fishers’ resource mapping and goliath grouper Epinephelus itajara (Serranidae) conservation in Brazil. Neotrop. Ichthyol. 7: 93-102. [ Links ]
Grandcourt, E.M., T.Z. Al Abdessalaam & F. Francis. 2006. Age, growth, mortality and reproduction of the blackspot snapper, Lutjanus fulviflamma (Forsskal, 1775), in the Southern Arabian Gulf. Fish. Res. 78: 203-210. [ Links ]
Grimes, C. 1987. Reproductive biology of the Lutjanidae: a review, p. 239-294. In J.J. Polovina & S. Ralston (eds.). Tropical Snappers and Groupers: biology and fisheries management. Westiew, Colorado, USA. [ Links ]
Grimes, C.B. & G.R. Huntsman. 1980. Reproductive biology of the vermillion snapper, Rhomboplites aurorubens, from North Carolina and South Carolina. Fish. B‑NOAA 78: 137-146. [ Links ]
Kaunda-Arara, B. & M.J. Ntiba. 1997. The reproductive biology of Lutjanus fulviflamma (Forsskal, 1775) in Kenyan inshore marine waters. Hydrobiologia 353: 153-160 [ Links ]
Kritzer, J.P. 2004. Sex-specific growth and mortality, spawning season, and female maturation of the stripey bass (Lutjanus carponotatus) on the Great Barrier Reef. Fish. B‑NOAA 102: 94-107. [ Links ]
Newman, S.J., M. Cappo & D.M. Williams. 2000. Age, growth, mortality rates and corresponding yield estimates using otoliths of the tropical red snappers, Lutjanus erythropterus, L. malabaricus and L. sebae, from the central Great Barrier Reef. Fish. Res. 48: 1-14. [ Links ]
Nikolsky, G.V. 1963. The ecology of fishes. Academic, London, United Kingdom. [ Links ]
Resende, S.M., B.P. Ferreira & T. Frédou. 2003. A Pesca de lutjanídeos no Nordeste do Brasil: histórico das pescarias, características das espécies e relevância para o manejo. Bol. Téc. Cient. CEPENE 11: 56-63. [ Links ]
Rojas, J.R. 1997. Fecundidad y épocas de reproducción del pargo mancha Lutjanus guttatus (Pises: Lutjanidae) en el Golfo de Nicoya, Costa Rica. Rev. Biol. Trop. 44/45: 477-487. [ Links ]
Rojas, R.L., F. Mejía-Arana, J.A. Palacios & K. Hiramatsu. 2009. Reproducción y crecimiento del pargo mancha Lutjanus guttatus (Pisces: Lutjanidae) en el Golfo de Nicoya, Costa Rica. Rev. Biol. Trop. 57: 125-131. [ Links ]
Sadovy, Y.J. 1996. Reproduction of reef fishery species, p. 15-59. In N.V.C Polunin & C.M. Roberts (eds.). Reef fisheries. Chapman & Hall, London, United Kingdom. [ Links ]
Santamaría-Miranda, A., J.F. Elorduy-Garay, M. Villalejo- Fuerte & A.A. Rojas- Herrera. 2003. Desarrollo gonadal y ciclo reproductivo de Lutjanus peru (Pisces: Lutjanidae) en Guerrero, México. Rev. Biol. Trop. 51: 489-502. [ Links ]
Silveira, M.P., J.C. Cousin & M. Haimovici. 1995. Estrutura ovárica e testicular do linguado Paralichthys orbignyanus (Valenciennes, 1839). Atlântica 17: 135- 152. [ Links ]
Sousa-Júnior, V.B., J.R. Feitosa & R. Salles. 2008. Ovarian analysis of the lane snapper, Lutjanus synagris (Actinopterygii: Lutjanidae), and considerations about its reproduction in Ceará State. Arq. Ciên. Mar 41: 79-84. [ Links ]
Vazzoler, A.E.A. de M. 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM, Maringá, Brasil. [ Links ]
Zar, J.H. 1996. Biostatistical analysis. Prentice-Hall, New Jersey, USA. [ Links ]
Internet Reference
IUCN. 2009. IUCN red list of threatened species, Geneva, Switzerland. (Downloaded: October 7, 2009, www.iucnredlist.org). [ Links ]